-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(12): 866-870
doi:10.5923/j.ajmms.20211112.05
Received: Nov. 6, 2021; Accepted: Nov. 22, 2021; Published: Dec. 15, 2021

Polymorphism of the MDR1 Gene as a Marker of Personification of Pharmacotherapy of Chronic Gastritis
Ochilova Gulrukh Saidovna
Bukhara State Medical Institute Named after “Abu Ali Ibn Sino”, Department of Pharmacology and Clinical Pharmacology, Uzbekistan
Correspondence to: Ochilova Gulrukh Saidovna, Bukhara State Medical Institute Named after “Abu Ali Ibn Sino”, Department of Pharmacology and Clinical Pharmacology, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

To predict the pharmacokinetic interaction of drugs in the process of absorption, distribution and excretion, it is also necessary to know the potential of substances to influence the body's transport systems, including glycoprotein-P (Pgp) and change its functional activity. This will eliminate the ineffectiveness of pharmacotherapy and undesirable reactions of drugs associated with changes in their concentration in blood plasma. The article presents the results of a genetic study of patients with chronic gastritis in the Bukhara region by polymorphism rs1045642 of the MDR-1 gene by polymorphic marker C3435T. It turned out that patients with the CT genotype prevail in this region and type B of chronic gastritis prevails in terms of occurrence. When studying the effectiveness of classical pharmacotherapy used, depending on the genotype of a patient with сhronic gastritis, the disease ends with recovery mainly in patients with the SS genotype.
Keywords: Pharmacogenetics, Multidrug-resistance gene, P-glycoprotein, Gene polymorphism, MDR-1 gene, CYP2C19 gene, Chronic gastritis, Polymorphic marker C3435T of the MDR1 gene, Personification of pharmacotherapy
Cite this paper: Ochilova Gulrukh Saidovna, Polymorphism of the MDR1 Gene as a Marker of Personification of Pharmacotherapy of Chronic Gastritis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 12, 2021, pp. 866-870. doi: 10.5923/j.ajmms.20211112.05.
1. Introduction
- Pharmacogenetics is one of the fairly young areas of pharmacology, which allows the doctor to select treatment taking into account genetic characteristics, that is, individual treatment is the personification of pharmacotherapy. Therefore, modern world health systems are "sick” with personalized medicine and colossal scientific research is being carried out in order to identify the influence of genes, their alleles and polymorphisms on the effectiveness of treatment with the help of genetic markers indicating the final result of pharmacotherapy of a particular disease [1,6,10]. In this regard, the studies carried out on the basis of the assessment of the informativeness of genetic markers are of interest.The development of pharmacology of the present century is impossible without taking into account the individual characteristics of the patient's genetics. The sequencing of the first human genome and the rapid development of technologies that followed it, which caused a significant reduction in the cost of genetic analysis and accelerated the timing of its implementation, made it possible to widely introduce methods of genetic diagnostics in scientific research and in practical medicine. Pharmacogenetics is still the basis for solving such problems [5,17].Genes, as in all spheres of the body's vital activity, remain the determining factor in the course of the process. Similarly, in the pharmacotherapy of specific nosological units, the pharmacodynamics and pharmacokinetics of each drug are dictated by the polymorphism of specific genes. In this regard, the tactics of treating pathological changes in such processes of the body should be carried out taking into account the genetics of each patient and it is necessary to recognize the presence of genes that carry out an individual pharmacological response of the body to the action of drugs [16].The tactics of treatment, taking into account the genetic characteristics of the body, dictates the personification of pharmacotherapy, that is, the actual issue of this direction is the individualization of treatment. The identification of allelic variants of each gene affecting the pharmacodynamics or pharmacokinetics of drugs used for therapy should be considered as the main factor in optimizing treatment (dose selection, routes of administration and replacement drug), as well as its effective and safe use [13].It was revealed that the MDR-1 gene is one of the main genes affecting the effectiveness of pharmacotherapy. The MDR-1 gene (multidrug-resistance gene) encodes P-glycoprotein (P-gp), which, located in the cytoplasmic membrane of various cells, performs the function of an ATP-dependent pump and promotes the excretion of various xenobiotics outside the cell [5]. Therefore, the expression of the MDR-1 gene contributes to the resistance of the cell to the drug used and plays an important role in the effectiveness of therapeutic measures. Namely, the protein P-glycoprotein encoded by the MDR-1 gene dictates the activity of the drug absorption process, being in the membrane of such normal cells of the body as epithelial cells lining the small and large intestines, pancreatic duct, bile tubules of the liver, proximal tubules of the kidneys and adrenal glands, on the ciliated epithelium of the lungs, in the trachea and bronchi, as well as in endotheliocytes of such histogematic barriers, as hematoencephalic, hematoovarial, hematotesticular. In addition, P-glycoprotein is found on the endothelium of lung vessels, in alveolar macrophages, in T- and B-lymphocytes, and even in the placenta. It is believed that P-glycoprotein prevents the absorption of drugs through the cell membrane or when they enter the cell - contributing to their speedy elimination, protects the body from xenobiotics. In the digestive system, P-glycoprotein in the role of a specific pump "pulls" the drug from the cell into the intestinal cavity. In liver hepatocytes, P-glycoprotein is involved in pumping out xenobiotics and releasing them into bile. In the epithelium of the renal tubules, P-glycoprotein promotes the active secretion of xenobiotics into the urine [9,14]. In endotheliocytes of histohematic barriers, P-glycoprotein inhibits the passage of xenobiotics into the central nervous system, into the testes, into the ovaries and through the placenta. The more toxins enter or form in a human cell, the faster the transcription and transmission of genes encoding this protein proceeds. Thus, by inhibiting the absorption and accelerating the excretion of xenobiotics, P-glycoprotein becomes a defender of the body [4]. It was found that the expression of the MDR-1 gene in men is 2.4 times greater than in women, which indicates the peculiarities of the pharmacokinetics of the drug depending on gender [12].The modern scientific literature describes one of the polymorphisms of the MDR-1 gene, which is formed due to a "silent" mutation in exon 26 at position 3435 (C3435T) - the replacement of a cytosine nucleotide with a thymidine one in the promoter zone of the MDR-1 gene [3].As described in the literature (Hoffmeyer S. et al., 2000), in homozygotes for the TT allele, the expression of the MDR-1 gene in the small intestine was more than 2 times less than the expression of the MDR-1 gene in SS homozygotes (p=0.056), which indicates a higher activity of P-glycoprotein in individuals with the SS genotype. During the subpopulation analysis, it was revealed that the activity of P-glycoprotein in Europeans was higher in carriers of the CC genotype, and in Japanese - in carriers of the TT genotype.It should also be noted that drugs entering the body are mainly metabolized in the liver under the influence of cytochrome P-450.Cytochrome P-450 is a protein complex with covalently bound heme (metalloprotein) that provides oxygen attachment. The number 450 indicates that the reduced heme associated with CO differs in the maximum absorption of light at a wavelength of 450 nm [8]. The cytochrome P450 complex (referred to as CYP450 in the literature) is involved in drug metabolism. All isoforms of cytochrome P-450 are grouped into the CYP1, CYP2, and CYP3 families [1,7]. Subfamilies A, B, C, D, and E are distinguished within the families. Within the subfamilies, isoforms are designated by an ordinal number, in the form of CYP2C19 - this is the name of the 19th cytochrome of the subfamily "C", family "2". In total, there are about 250 different types of cytochrome P-450, of which about 50 are in the human body and only 6 of them (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4) are related to drug metabolism [2,11]. The most significant, according to modern concepts, are changes in pharmacokinetics in the metabolism of drugs with the participation of cytochromes.In our studies, the object of study was acid-dependent diseases of the digestive system, where proton pump inhibitors are first-line drugs. More and more data are accumulating in the literature that the therapeutic effect of proton pump inhibitors significantly depends on the rate of drug excretion from the body. Since the metabolism of proton pump inhibitors occurs mainly in the liver with the participation of CYP2C19, the polymorphism of the genes of the cytochrome C2C19 system is a determining factor that the rate of onset, duration of the antisecretory effect of proton pump inhibitors and manifestations of side effects in patients differ significantly [18].The literature describes that in the Russian population, the prevalence of mutations of the CYP2C19 gene encoding the metabolism of proton pump inhibitors had an effect on the pharmacokinetics of drugs in the following way: homozygotes, no mutations – rapid metabolism of proton pump inhibitors; heterozygotes, one mutation; two mutations - slow metabolism of proton pump inhibitors, respectively. Thus, it turns out that from 8.3 to 20.5% of patients are resistant to a single dose of proton pump inhibitors [15,19].Thus, the MDR1 gene and CYP2C19 are the main factors that ensure the metabolism of proton pump inhibitors, as well as the effectiveness of pharmacotherapy in general [20].However, there are no studies on the influence of allelic variants of the CYP2C19 gene and polymorphisms of the MDR1 gene, that is, the patient's genotype on the effectiveness of treatment of chronic gastritis in patients living in the Bukhara region, which was the basis for this study.
2. Materials and Methods
- The age of patients with chronic gastritis ranged from 18 to 63 years. At the same time, it should be noted that women predominated among patients with chronic gastritis.The initial stage of our work was the selection and optimization of the system of oligoprimes for the detection of polymorphism rs1045642 of the MDR-1 gene by polymorphic marker C3435T and polymorphism rs4244285 of the SUR2C19 gene by polymorphic marker G681A, i.e. improvements in the methodological method for detecting these genetic markers. Nucleotide sequences of detection of polymorphism rs1045642 of the MDR-1 gene and polymorphism rs4244285 of the SUR2C19 gene were selected using the program "Oligo v.6.31" (Molecular Biology Insights Inc., USA) and synthesized in LLC "Syntol" and NPF "Litech” (Moscow).The remaining components were purchased from the world's leading manufacturers - Serva (Germany), Sigma (USA), Helicon NPFLitech”, Sibenzim (Russia), etc.The adaptation of primer systems for standard PCR analysis was carried out using PCR analyzers “Appliedbiosystems 2720” (USA) and Rotor-Gene 6000 (Corbett Australia). For amplification, a reaction mixture with a volume of 25 µl was used, which contained 2.5 µl of 1 OxTaq buffer (67 mMtris-HCl (pH 8.8), 16.6 mM (NH4)2S04>, 2.5mm MgCl2, 0.01% Tween-20), 0.1 µg of genomic DNA, a mixture of dNTP (dATP, dGTP, dCTP, dTTP of 200 µm each), 1 unit. Termusaquaticus DNA polymerase (manufactured by Silex, Moscow) and 5-10 pM locus-specific oligonucleotide primers. The temperature-time parameters were changed depending on the pairs of oligoprimes.To detect rs1045642 of the MDR-1 gene and rs4244285 of the SUR2C19 gene: preliminary denaturation - 940C (1 min. 1 cycle), 35 amplification cycles: 930C (10 sec.) - denaturation, 640C (10 sec.) - annealing of primers, 720C (20 sec.) - elongation, and final synthesis 720C (1 min. 1 cycle), 10 min storage.Polymorphic regions of the MDR1 gene and the SUR2C19 gene were detected using PCR-SSP.These primers were constructed using Vector NTI 7.1 and Primo software packages [69]. The amplification mixture (10 µl) contained 70 mM Tris-HCl (pH 9.0), 20 mM (NH4)2 SO4, 1.0 mM MgCl2, 0.025% Twin-20, 0.025% NP-40, 5 pmol of each primer, 0.2 mM dNTP, 0.5 units. Taq polymerases and 100-200 ng DNA, mineral oil. Amplification program: 95°C, 5 min. Then 10 cycles: 95°C – 1 min, 64°C – 1 min, 72°C – 1 min; and 20 cycles: 95°C – 30 s, 58°C – 50 s, 72°C – 50 s. The specificity and number of amplified fragments were checked by electrophoresis in agarose gel.When carrying out amplification with DNA samples with a standard concentration (80-100 ng/ml), we obtained "false-positive" results, compared with the positive control. After diluting the samples with a TE buffer to obtain a concentration of 20-60ng/ml, amplification fragments were detected in all reactions, compared with the standard set. These results allow us to officially enter information about the level of DNA used (20-60 ng/ml) in the methodological manual for PCR studies of polymorphisms rs1045642 of the MDR-1 gene and rs4244285 of the SUR2C19 gene.A comparative analysis of 50 samples of control DNA established a positive correlation between our results and the data obtained by the standardized test system of PF Litech (Moscow). Heterozygous and homozygous genotypes were detected in the same DNA samples, the negative result was confirmed by both methods (high comparability of results). The revealed minor differences were statistically insignificant (P>0.05).
3. Result and Discussion
- When studying the polymorphism rs1045642 of the MDR1 gene by polymorphic marker C3435T in patients with chronic gastritis living in the Bukhara region (Fig. 1), the CT genotype prevails (59%).
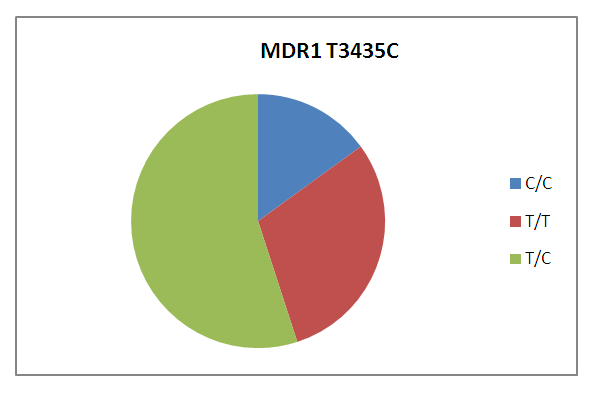 | Figure 1. Frequency of distribution of genotypes of polymorphism C3435T of the MDR1 gene in patients with chronic gastritis, regardless of the type of gastritis |
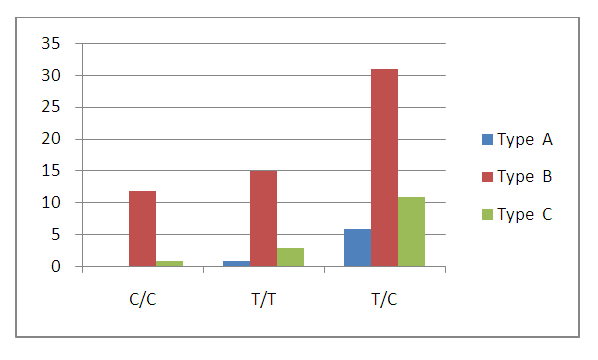 | Figure 2. Distribution of types of chronic gastritis depending on the genotypes of polymorphism C3435T of the MDR1 gene |
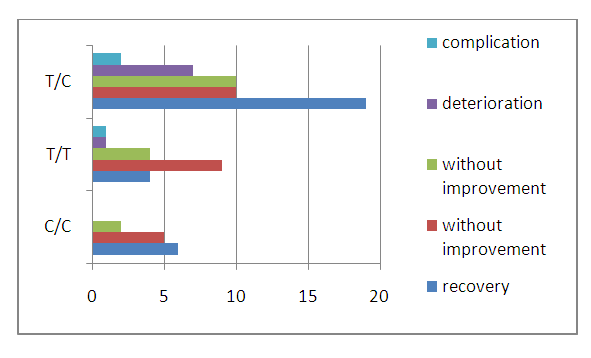 | Figure 3. Результаты лечения хронического гастрита и их взаимосвязь с частотой распределения генотипов полиморфизма С3435Т гена MDR-1 при хроническом гастрите |
4. Conclusions
- Thus, the research results show that in order to obtain a complete pharmacotherapeutic effect, the doctor needs to have information about the patient's genotype. Such patient data helps the doctor to optimize the chosen treatment plan and, most importantly, to select the dose and treat the patient effectively and safely. Since the human genetic apparatus is individual, unique, we believe that such information about the patient contributes to the individualization of treatment, that is, the personification of pharmacotherapy, which will serve as the basis for safe and highly effective treatment, which in modern medicine is considered relevant and a requirement of the time.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML