-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(11): 830-834
doi:10.5923/j.ajmms.20211111.18
Received: Nov. 9, 2021; Accepted: Nov. 20, 2021; Published: Nov. 28, 2021

Efficiency of Gum Resin Ferula Asafetid in Correction of Disturbances of the Liver in Acute Paracetamol-Induced Hepatitis in Rats
Khakimov Z. Z. 1, Tulyaganov S. X. 2, Rakhmanov A. X. 1, Safaeva Sh. T. 3
1Tashkent Medical Academy, Tashkent, Uzbekistan
2"ABDU-S" Company, Tashkent, Uzbekistan
3Urgench Branch of the Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Rakhmanov A. X. , Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Acute toxic hepatitis induced by paracetamol (APIH) in adult white male rats 180-200 g., experimental therapy with gum-resin Ferula asafoetida (GRFA) at doses 50 mg/kg led to a clear elimination of reduced bile secretion, assessed by the intensity of exocrine liver function and the chemical composition of bile, in which bile secretion increased by 101.7%, quantity of bile acids by 46.6%), cholesterol by 54.6% and bilirubin by 56.1%. The doubling of the dose of the medicine (100 mg/kg) did not cause to increase the effect. The positive therapeutic effect of GRFA is also confirmed in the results of the study of liver function tests in blood serum. The medicine eliminated the phenomena of hypoproteinemia, hyperbilirubinemia, cytolytic and cholestatic syndromes in rats with acute drug-induced hepatitis. At the same time, GRFA surpasses the reference hepatoprotector - Legalon by its pharmacological activity.
Keywords: Acute drug hepatitis, Paracetamol, Bile, Liver function tests
Cite this paper: Khakimov Z. Z. , Tulyaganov S. X. , Rakhmanov A. X. , Safaeva Sh. T. , Efficiency of Gum Resin Ferula Asafetid in Correction of Disturbances of the Liver in Acute Paracetamol-Induced Hepatitis in Rats, American Journal of Medicine and Medical Sciences, Vol. 11 No. 11, 2021, pp. 830-834. doi: 10.5923/j.ajmms.20211111.18.
Article Outline
1. Introduction
- Liver damage by the influence of various etiological factors is one of the most common pathologies of the hepatobiliary system. In order to restore the functional state of the liver, the substances using in practical medicine do not always satisfy clinicians [1]. In this regard, searching for and developing new compounds with a high therapeutic effect in liver pathology is an actual task of pharmacologists. Literature data indicate that even in highly developed countries, drugs with a hepatotoxic effect are taken without permission, without appropriate supervision of medical personnel [2,3].Since the vast majority of drugs are eliminated by hepatocytes, an increase in the hepatotoxicity of drugs can be expected. Among the latter, paracetamol occupies one of the important places due to its different pharmacodynamic effect (antipyretic, analgesic) [4,5,6,7]. However, its metabolite N - acetyl - n - benzoquinone imine, which binds glutathione, exhibits hepatotoxic, nephrotoxic effects (necrosis of hepatocytes and renal tubules). At the same time, the drug is widely used in pediatric practice as an analgesic and antipyretic agent. In economically developed countries, such as the USA, Great Britain, paracetamol is the most common reason of acute liver failure [6,8,9].The aim of this work was to study the effect of gum- resin Ferula asafoetida (GRFA) on the functional state of the liver in acute hepatitis induced by paracetamol in comparison with the well-known hepatoprotector Legalon. The functional state of the liver was judged by the intensity of the exocrine function of the liver, the chemical composition of bile and biochemical parameters of the blood ("liver tests").
2. Material and Methods
2.1. Experiments
- The technology of obtaining gum-resin from plants Ferula asafetida L.. (GRFA) was described by us in previous studies [10,11].The experiments were carried out on sexually mature male rats with an initial weight of 180-200 g. The maintenance of laboratory animals corresponded to the sanitary rules for the design, equipment and maintenance of experimental biological clinics. Feeding was carried out with natural and briquetted food, in accordance with the norms. After passing 14 days of quarantine, several groups of animals were formed, 6 animals in each, taking into account body weight. The experiments were carried out in accordance with the rules of a good laboratory practice (GLP) for preclinical research, as well as the rules and International Recommendations of the European Convention for the Protection of Vertebrate Animals used in Experimental Research (1986). The approval of Ethic Committee of Republic of Uzbekistan was taken before beginning of the experiment (protocol No 6/17-1579, 23/09/2021).Acute drug-induced hepatitis was reproduced by intragastric administration of paracetamol at a dose of 1500 mg/kg once a day for two days [12]. One day after the last administration of paracetamol, experimental therapy was carried out for six days. For this, GRFA was administered at doses of 50 and 100 mg/kg, and Legalon - 100 mg/kg. 24 hours after the final administration of medicines, the bile excretion function of the liver was investigated by inserting a polyethylene catheter into the common bile duct of anesthetized animals (intraperitoneal administration of sodium ethaminal at a dose of 50 mg/kg) [13]. The choleric activity of various doses of GRFA and Legalon was judged by the total amount of excreted bile for 4 hours of the experiment, as well as by the concentration and amount of its components: bilirubin, cholesterol and bile acids [14,15]. In hourly portions, the concentration (mg%) and the total amount (mg per 100 g of body weight) of bile acids, cholesterol and bilirubin were determined [15,16].In the second series of experiments, various biochemical markers characterizing liver function were determined in the blood according to the "Guidelines for conducting preclinical studies of drugs" [17]. Determination of the amount of total protein, albumin, total bilirubin, as well as the activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT) and alkaline phosphatase (ALP) in blood serum was carried out photometrically on a semi-automatic biochemical analyzer (China, 2014 Mindray) using kits from Human (Germany) and Cypress diagnostics (Belgium).
2.2. Statistical Analysis
- The data obtained were processed by the method of variation statistics using the paired Student's test and one-way analysis of variance using the standard software package BIOSTAT 2009 with an assessment of the significance of indicators (Mean±Std error). Differences in the compared groups were considered significant at a significance level of 95% p < 0.05, in the case of 0.05 < p < 0.10, the differences were assessed as a trend.
3. Results and Discussion
- Paracetamol had a expressed hepatotoxic effect in the used dose in rats. So, the exocrine function of the liver was significantly reduced by more than two times under influence of paracetamol and there were an almost two times decreasing of quantity of chelates (by 41.1%), cholesterol (by 44.5%) and bilirubin (by 50.7%) in the excreted bile. These data confirm the results of our earlier studies [18]. Consequently, paracetamol caused a significant depression of the functional state of the liver in rats, which was assessed by the specific bile excretory function of liver (Table 1).
|
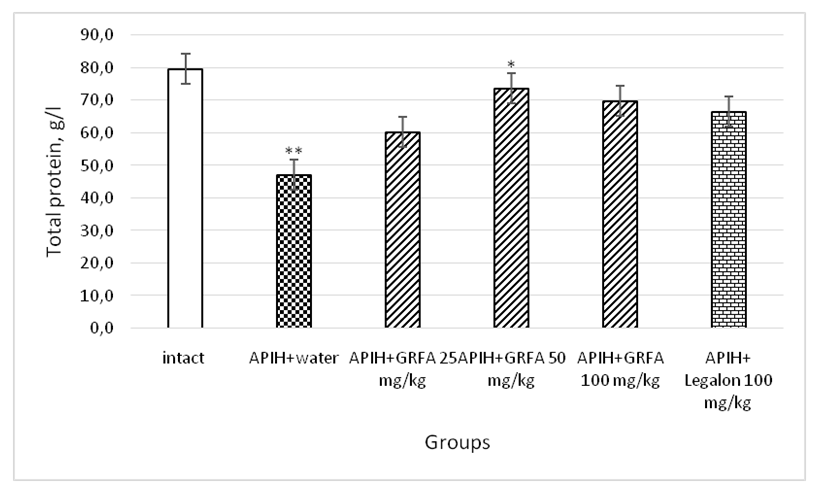 | Picture 1. Influence of GRFA and Legalon on the total protein content in blood serum in rats with acute paracetamol-induced hepatitis. * – statistically significant in comparison with control group, ** - statistically significant in comparison with intact group |
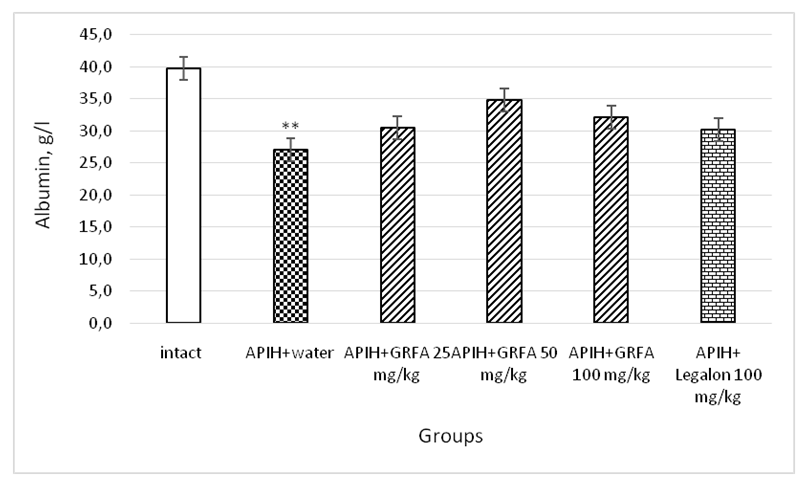 | Picture 2. Influence of GRFA and Legalon on the content of albumin in the blood serum of rats with acute paracetamol-induced hepatitis. * – statistically significant in comparison with control group, ** - statistically significant in comparison with intact group |
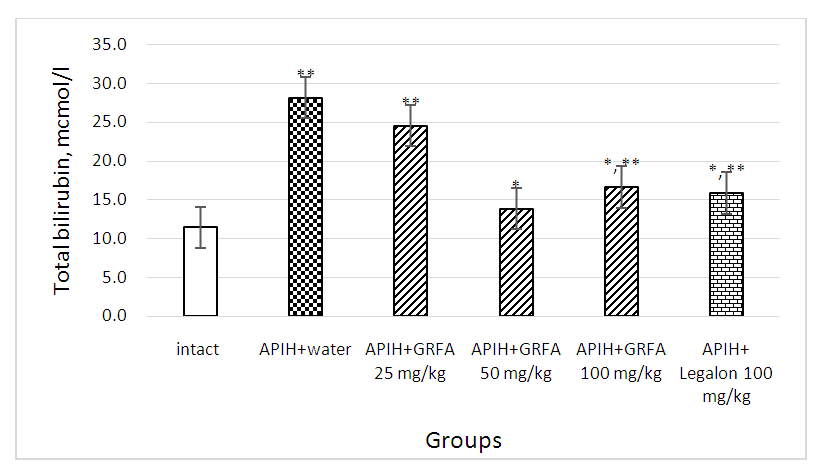 | Picture 3. Influence of GRFA and Legalon on the content of total bilirubin in blood serum in rats with acute paracetamol-induced hepatitis. * – statistically significant in comparison with control group, ** - statistically significant in comparison with intact group |
4. Conclusions
- 1. GRFA clearly enhances the exocrine function of the liver and the chemical composition of bile in rats with acute paracetamol- induced hepatitis.2. The therapeutic effect of GRFA is also confirmed in the results of biochemical blood tests, which indicate the elimination of cytolytic and cholestatic syndromes.3. Judging by the increase in the excretion of bilirubin in the composition of bile and the decrease in its concentration in the blood serum, it can be stated that the GRFA has a hypobilirubinemic effect.4. GRFA, with therapeutic use, eliminates hyproproteinemia in rats with drug-induced hepatitis.
References
| [1] | Matveev A.V., 2013, Hepatoprotectors. Analysis of international studies on drugs in the group of drugs for the liver, IT "ARIAL", Simferopol, 384. |
| [2] | Karateev A.E., Nasonov E.L., Ivashkin V.T., 2018, Rational use of non-steroidal anti-inflammatory drugs. Clinical guidelines, Scientific and practical rheumatology, 56, 1, 1-29. |
| [3] | Zhuravleva M.V., Kukes V.G., Prokofiev A.B., 2016, Rational use of NSAIDs - balance of efficacy and safety (literature review), International Journal of Applied and Fundamental Research, 6, 4, 687-696. |
| [4] | Zvyagintseva T.D., Chernobay A.I., 2014, Chronic liver diseases: focus on polycomposite plant hepatoprotectors and antioxidants, Suchasna gastroenterologiya, 4, 70-76. |
| [5] | Drozdov V.N., Bagdasaryan A.A., Serebrova S.Yu., 2019, The optimal choice of analgesic and antipyretic drugs in pediatric practice, Medical Council, 2, 106-112. |
| [6] | Ivashkin V.T., Baranovsky A.Yu., Raikhelson K.L., 2019, Medicinal liver damage (clinical guidelines for doctors), Russian Journal of Gastroenterology, Hepatology, Colopractology, 29, 1, 101-132. |
| [7] | Klimov L.Ya., Aksyonov A.G., Popova E.V., 2018, Fulminant hepatic failure while taking acetaminophen, Medical Council, 11, 76-83. |
| [8] | Pashko A.Yu., 2014, Dose-dependent hepatotoxic effect of paracetamol and its correction by the combination of taurine with zinc diaspartate, Collection of scientific papers "BSMU: 90 years at the forefront of medical science and practice.", Minsk, 224-226. |
| [9] | Bunchorntavakul C., Reddy K.R., 2013, Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther., 37, 1, 3-17. |
| [10] | KhakimovZ. Z., Rakhmanov A. Kh., Safaeva Sh. T., 2020, Hepatoprotective Activity of Gum Resin of Ferula Assa-Foetida. American Journal of Medicine and Medical Sciences, 10, 9, 728-732. |
| [11] | Tulyaganov S.A., Nabiev A. A., method of obtaining an agent with anti-inflammatory and prostatoprotective action, Patent UZ IAP. 5453, Registered in Tashkent on August 24, 2017. |
| [12] | Khakimov Z.Z., Rakhmanov A.Kh., Safaeva Sh.T., Kurbanova N.N., 2021, Comparative study of hepatoprotective activity of gum resin ferula asaphetid and legalon in acute toxic hepatitis induced by Paracetamol. Journal of Biomedicine and Practice., 6, 1, 232-238. |
| [13] | Khakimov Z.Z., Rakhmanov A.Kh., Mavlanov Sh.R., 2021, The effectiveness of a mixture of extracts of medicinal disorders in the correction of violations of the functional state of the liver in its lesions of various etiology. Tashkent, 156. |
| [14] | Mavlanov Sh.R., Khakimov Z.Z., Rakhmanov A.Kh, 2017, Yangi pharmacologic faol birikmalarni hepato-biliar tisim faolytiga ta'sirini experimental urganish usullari. Tashkent, 63. |
| [15] | Khakimov Z.Z., Akramova Ya.Z., Makhmudov S.S., 2018, The effectiveness of interferon inducers in the correction of the functional state of the liver in toxic getatitis. Tashkent, 118. |
| [16] | Khakimov Z.Z., Makhmudov S.S., 2011, Gossypol polymer composition siningexperimental, 1, 99-101. |
| [17] | Mironov A.N., 2012, Guidelines for the conduct of preclinical studies of medical supplies. Part One. -M.: GrifiK, 944. |
| [18] | Khakimov Z.Z., Rakhmanov A.Kh., Safaeva Sh.T., 2020, Comparative study of the prophylactic effect of various doses of gum resin Ferula asafoetid and Legalon on the biliary function of the liver in acute paracetamol hepatitis. RE-HEALTH Journal, 4, 88-92. |
| [19] | Safaeva Sh. T., Khakimov Z. Z., Rakhmanov A. Kh., Khakimova D. Z., 2020, Effectiveness of gum resin ashaphetide ferula in correction of external secretary function of the liver in acute toxic hepatitis, J. Science of Europe (Praha, Czech Republic), 1, 57, 21-24. |
| [20] | Khakimov Z.Z., Rakhmanov A.Kh., Safaeva Sh. T., 2021, Asphervon-related increase of bile secretion as a preventive measure and therapeutic agent for heliotrin induced hepatitis in rats. Natl J Physiol Pharm Pharmacol, 11, 1, 96-101. |
| [21] | Kishkin A.A., 2019, Clinical laboratory diagnostics: textbook, M., GEOTAR-Media - 2nd ed., 837. |
| [22] | Amalraj A., Gopi S., 2017, Biological activities and medicinal properties of Asafoetida: A review, Journal of Traditional and Complementary Medicine, 7, 347-359. |
| [23] | Chandran S., Sakthivel M., Thirumavalavan M., 2017, A facile approach to the isolation of proteins in Ferula asafoetida and their enzyme stabilizing, anti-microbial and antioxidant activity, Int. J. Biol. Macromol, 102, 1211-1219. |
| [24] | Dyakov A.A., Perfilova V.N., Tyurenkov I.N., 2005, Antiarrhythmic action of ferulic acid, Bulletin of arrhythmology, 39, 49-52. |
| [25] | Khakimov Z.Z., Rakhmanov A.Kh., Safaeva Sh.T., 2021, Experimental substantiation of the hepatoprotective activity of Asfervon, Guidelines, Tashkent, 16. |
| [26] | Khakimov Z.Z., Rakhmanov A.Kh., Tulyaganov S.Kh., 2018, Preclinical study of the safety of Asfervon, Pharmaceutical Bulletin of Uzbekistan, 1, 69-74. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML