-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(11): 791-794
doi:10.5923/j.ajmms.20211111.10
Received: Oct. 28, 2021; Accepted: Nov. 15, 2021; Published: Nov. 18, 2021

The Use of AFP and PIVKA II for Screening Hepatocellular Carcinoma in Patients with Viral Cirrhosis
Krestina Stepanovna Brigida, Aziza Saydullaevna Khikmatullaeva, Shakhnoza Khayrullaevna Akhmedova, Nozimakhon Rakhmatullakhodjaevna Turabova, Erkin Isakovish Musabayev
Research Institute of Virology of the Republican Specialized Scientific Practical Medical Center of Epidemiology, Microbiology, Infections and Parasitics Diseases of Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Krestina Stepanovna Brigida, Research Institute of Virology of the Republican Specialized Scientific Practical Medical Center of Epidemiology, Microbiology, Infections and Parasitics Diseases of Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hepatocellular carcinoma is the most common form of liver cancer. Secondary prevention of HCC is based on the introduction of screening programs for the detection of hepatocellular carcinoma. This article highlights the results of screening hepatocellular carcinoma among patients with cirrhosis of the liver of viral etiology using AFP and PIVKA II. Blood sampling for this study was carried out on the day of admission of the patient to hospital treatment at the Research Institute of Virology.Whole blood was collected from patients and collected in pre-labeled EDTA vacuum tubes. The exact volume was drawn into the test tube, avoiding overfilling of the test tubes. Not later than 2 minutes from the moment of filling, the contents of the tube were mixed by gentle inversion, avoiding foaming. Within 1 hour from the moment of sampling, centrifugation of blood samples was carried out in order to separate the plasma. Of 60 patients with cirrhosis of HCV etiology, 6 patients had PIVKA II and AFP was higher than normal. In 5 of them, a mass was visualized on ultrasound and in 1 on MSCT. An isolated increase in PIVKA II was found in 8 patients, in 4 of them a mass was detected on ultrasound, and in 4 it was detected using MSCT. In 5 patients in this group, an isolated increase in AFP was observed. In 1 of them, a mass was found on ultrasound, and 2 of 4 patients without a mass on ultrasound subsequently underwent MSCT examination, however, no mass was found, 2 patients refused further research. The introduction into routine practice of the determination of tumor markers in patients with cirrhosis of viral etiology will make it possible to detect hepatocellular carcinoma in the early stages, which will improve the survival rate and quality of life of patients.
Keywords: HBV, HCV, HDV, Hepatocellular carcinoma, Cirrhosis AFP, PIVKA II, Screening
Cite this paper: Krestina Stepanovna Brigida, Aziza Saydullaevna Khikmatullaeva, Shakhnoza Khayrullaevna Akhmedova, Nozimakhon Rakhmatullakhodjaevna Turabova, Erkin Isakovish Musabayev, The Use of AFP and PIVKA II for Screening Hepatocellular Carcinoma in Patients with Viral Cirrhosis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 11, 2021, pp. 791-794. doi: 10.5923/j.ajmms.20211111.10.
1. Introduction
- Hepatocellular carcinoma is the most common form of liver cancer. Secondary prevention of hepatocellular carcinoma is based on the introduction of screening programs to directly detect the hepatocellular carcinoma itself [1]. A number of cohort studies and the creation of models have demonstrated that screening for hepatocellular carcinoma is cost-effective and contributes to the improvement of early detection of tumor processes, therapy and survival, if the detection of tumors, cure rates and survival, with access to screening is higher than 34% of patients from risk groups [2-6]. In fact, access to screening for risk groups does not exceed 20% [7].Patient categories and screening methods are discussed in many publications, the main of which are the joint guidelines of the European Organization for Research and Treatment of Cancer and the American Association for the Study of Liver Diseases recommendations [8-11]. One of the widespread methods of screening for hepatocellular carcinoma is ultrasound liver, despite the fact that the sensitivity of the method varies, according to different authors, from 33 to 96% [12]. The identification of reliable serum biomarkers is of paramount importance in the diagnosis of liver cancer, especially for the early detection and screening of hepatocellular carcinoma. [13,14]. Serum tumor markers are most suitable for clinical routine assessment and population screening because these tests require very small amounts of serum, are cheap, have high reproducibility, and do not require specific pretreatment [15].
2. Materials and Methods
- The study was carried out at the Research Institute of Virology. Blood sampling was carried out in patients with cirrhosis of viral etiology at the Research Institute of Virology. The criteria for inclusion in the group of patients with cirrhosis of HBV, HBV + HDV and HCV etiology without hepatocellular carcinoma were age over 18 years, patient consent to participate in the study, and the presence of markers of viral hepatitis. The exclusion criteria were age under 18 years of age and refusal to study, histologically confirmed hepatocellular carcinoma.Blood sampling for this study was carried out on the day of admission of the patient to hospital treatment at the Research Institute of Virology.Whole blood was collected from patients and collected in pre-labeled EDTA vacuum tubes. The exact volume was drawn into the test tube, avoiding overfilling of the test tubes. Not later than 2 minutes from the moment of filling, the contents of the tube were mixed by gentle inversion, avoiding foaming. Within 1 hour from the moment of sampling, centrifugation of blood samples was carried out in order to separate the plasma. The primary tube with blood was centrifuged at 10,000 rpm for 5 min. At least 1 ml of serum was taken and transferred into 3 separate labeled Eppendorf tubes with a volume of 2 ml, while at least 200 μl of plasma was taken into each tube. Aliquots of blood serum samples with a volume of 200 μl were stored at a temperature not exceeding -80° until the moment of the study. If necessary, the samples were transported in a special thermal container on dry ice.The AFP-IFA-BEST set by Vector Best (Russia) was used to determine the AFP level. All samples were affixed to a Biotek spectrophotometer by ELISA. The PIVKA II 100 test kit from Maglumi (China) was used to determine the PIVKA II level. Determination of the PIVKA II level was carried out on a Maglumi 800 (Snibe). The analyzes were performed according to the instructions of the manufacturers.
3. Results and Discusion
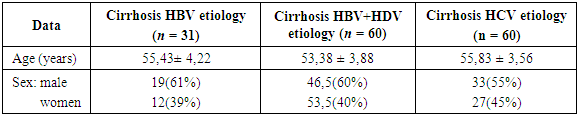
- The aim of the study was the early detection of hepatocellular carcinoma, for which screening was carried out among patients with cirrhosis of HBV, HCV, HBV + HDV etiology for hepatocellular carcinoma. The screening program included 151 patients with viral hepatitis cirrhosis. using tumor markers PIVKA II and AFP. The distribution by sex, age and etiological characteristics is presented in Table 1.
|
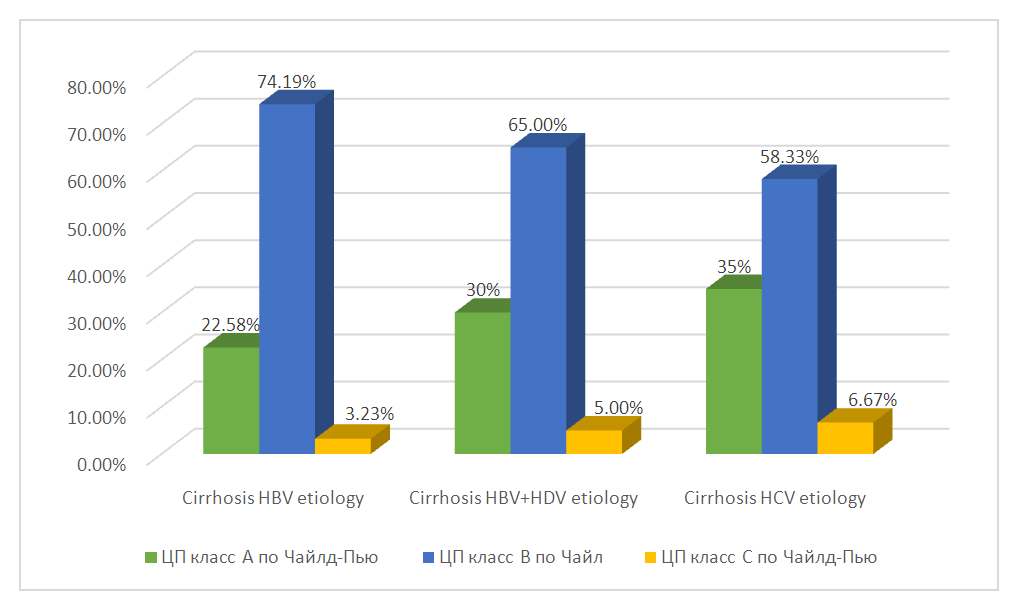 | Figure 1. Distribution of patients in the study groups according to the Child-Pugh classification |
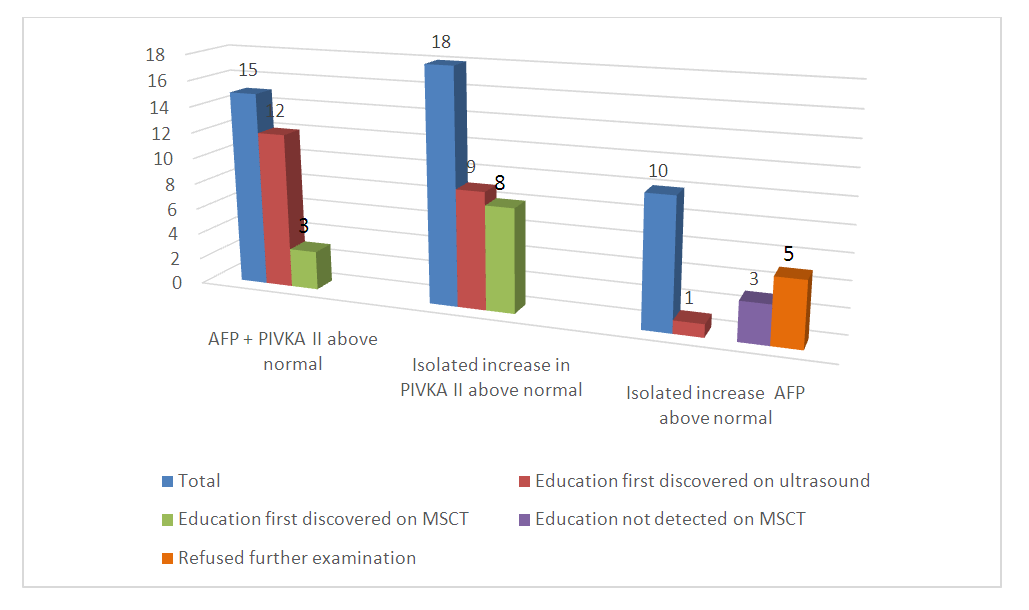 | Figure 2. Results of screening for hepatocellular carcinoma among patients with cirrhosis of viral etiology |
4. Conclusions
- The introduction into routine practice of the determination of tumor markers in patients with cirrhosis of viral etiology will make it possible to detect hepatocellular carcinoma in the early stages, which will improve the survival rate and quality of life of patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML