R. A. Ibadov1, G. Sh. Khamraeva2, S. Kh. Ibragimov1
1Republican Specialized Scientific and Practical Medical Center for Surgery named after Academician V. Vakhidov, Republic of Uzbekistan, Tashkent
2Center for the Development of Professional Qualifications of Medical Workers, Republic of Uzbekistan, Tashkent
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
From the first days of the outbreak of the new coronavirus COVID-19, the government of the Republic of Uzbekistan has been constantly monitoring the situation and taking timely response measures. The Ministry of Health works closely with international experts and partners to quickly generate scientific evidence, track its spread, assess virulence, and advise the public on measures to protect health and prevent the spread of the outbreak. The COVID-19 pandemic has led not only to congestion in healthcare systems, but has also created numerous challenges and challenges in understanding the pathogenesis of various manifestations of COVID-19, the solution of which is of paramount importance in the development of diagnostic and treatment algorithms. At the moment, there is no specific criterion that distinguishes between virus-associated lung injury and secondary bacterial pneumonia, and determines the need for antibiotic therapy, therefore, it is advisable to comprehensively evaluate the results of clinical, laboratory and instrumental examinations. The article provides an analysis of the results of bacteriological studies of cultures from the trachea, pleural fluid, sputum, etc. The developed algorithms for antibacterial therapy of patients with COVID-19 in the conditions of intensive care and intensive care units will significantly increase the effectiveness of optimal, etiotropic and pathogenetic therapy.
Keywords:
Antibiotic therapy, COVID-19, Emergency units,Zangiata Medical Center
Cite this paper: R. A. Ibadov, G. Sh. Khamraeva, S. Kh. Ibragimov, Antibiotic Therapy in Patients with COVID-19 in the Conditions of Intensive Care and Emergency Units, American Journal of Medicine and Medical Sciences, Vol. 11 No. 10, 2021, pp. 740-743. doi: 10.5923/j.ajmms.20211110.16.
1. Introduction
Currently, there is a principled position on the need to distinguish between the virus-associated lung damage ("viral pneumonia") and secondary bacterial pneumonia. Based on this concept, "viral pneumonia" can be of varying severity, up to ARDS, but will not require massive antibiotic therapy. At the same time, the addition of secondary bacterial pneumonia requires the immediate appointment of antibiotic therapy, considering the most likely pathogens (Staphylococcus aureus (MSSA, MRSA), Streptococcus pneumoniae, Hemophilus influenza), etc. According to data from previous epidemics of influenza (2009-2010) and outbreaks of coronavirus infection (2004, 2012), an increase in the detection rate of infection with Staphylococcus aureus, including MRSA [Draft Clinical Guidelines "Community-Acquired Pneumonia" 2018] was shown. At the moment, there is no specific criterion that distinguishes the virus-associated lung injury and secondary bacterial pneumonia, and determines the need for antibiotic therapy, therefore it is advisable to comprehensively evaluate the results of clinical, laboratory and instrumental examinations [Algorithm for prescribing antibiotic therapy 2020].
2. Purpose
To develop an algorithm for antibiotic therapy for patients with COVID-19 in the conditions of intensive care units.
3. Materials and Methods
The analysis of the results of bacteriological studies of cultures from the trachea, pleural fluid, bronchial lavage water, sputum of 300 patients with COVID-19 in the clinic of Zangiata Medical Center No. 1 was carried out. In all patients, the diagnosis was confirmed by virology (PCR). We studied the results with positive bacteriological cultures with the development of secondary bacterial pneumonia. All patients were divided into three groups: I - severe - 180, II - severe with concomitant pathology - 75 and III - in an extremely serious condition - 45 people.Identification of isolated microorganisms was carried out using test kits from Hi-Media, India, the determination of sensitivity to antimicrobial drugs was carried out by the disk-diffusion method in accordance with the NCCLS recommendations.The sensitivity of microorganisms to antibiotics: cephalosporins, aminoglycosides, tetracyclines, fluoroquinolones, carbapenems, glycopeptides, polymyxin, etc., as well as to antifungal drugs - was determined by diffusion into agar from discs.
4. Results
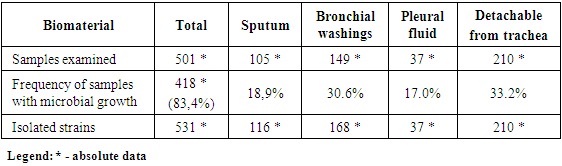
418 samples of clinical material were examined from positive analyzes from 300 patients, from which various types of microorganisms were isolated. 531 cultures of microorganisms were isolated from 418 samples (gram-negative flora - 64.7%, gram-positive flora - 14.3%, Candida spp. - 21%).Analysis of the study of various types of biomaterials revealed the seeding rate: positive growth of microorganisms - 83.4%. The frequency of excretion of pathogens from sputum was 18.9%, bronchial lavage water - 30.6%, from the pleural cavity - 17.0%, from the trachea - 33.2%. In 27% of cases, pathogens were isolated associated (Table 1).Table 1. Frequency of samples with microbial growth
 |
| |
|
Pathogens isolated in the studied groups were distributed: in the first group (out of 188 positive samples) - 196 strains of pathogens, in the second group (out of 124 positive samples) - 187 strains of pathogens, in the third group (out of 106 positive samples) - 148 strains of pathogens ...From the isolated strains, gram-negative flora (64.7%) prevailed; Klebsiella pneumonia - 27.0%, Klebsiella spp. - 12.0%, Escherichia coli - 7.8%, Pseudomonas aeruginosa - 4.0%, Enterobacter spp. - 2.8%, Stenotrophomons maltophilia - 1.1%. Gram-positive flora (14.3%): Staphylococcus aureus - 6.3%, Staphylococcus spp. - 6.1%, Streptococcus spp. - 4.7%, Streptococcus pneumonia - 2.8%, Enterococcus spp. - 1.5%, Staphylococcus epidermidis - 1.3%, Hemophilus influenza - 0.7%, fungi of the genus Candida spp. occurred in 21.0% of cases (Fig. 1).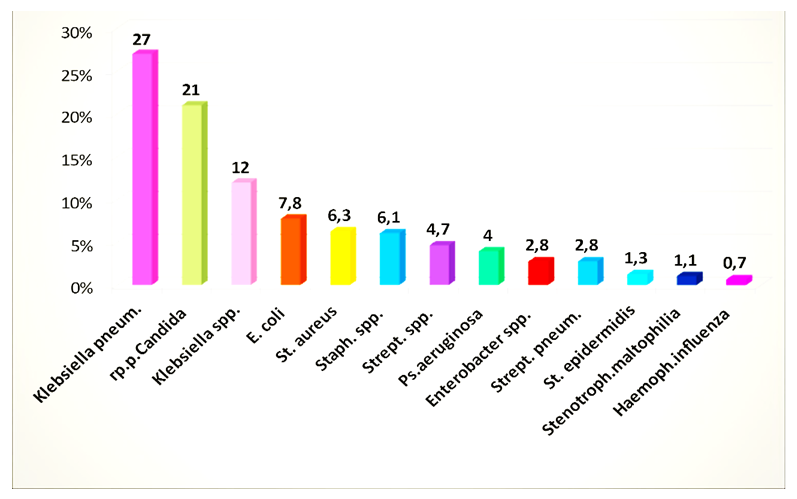 | Figure 1. Microorganisms isolated from patients with COVID-19 pneumonia in the ICU |
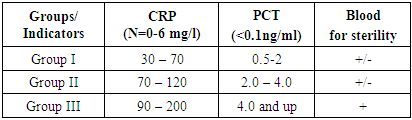
Analysis of antibiogram of isolated cultures in patients with severe pneumonia shows high resistance to a wide range of antibiotics, namely: Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, had high resistance to all antibiotics, except for imipenem (7% R strains), polymyxin (2% R strains), resistance to meropenem was 37% of resistant strains, cefoperazone / sulbactam and piperacillin / tazobactam - 67-75%, to cephalosporins was III generation - 87-95%, ciprofloxacin, ofloxacin, levofloxacin - 79-94% amikacin - 67%, doxycycline - 92%. Staphylococcus aureus in 34% of cases was sensitive to third generation cephalosporins (cefotaxime, ceftriaxone, cefoperazone), cefoperazone / sulbactam in 27% of cases, to tetracyclines - 36.3% of resistant strains. There were no strains resistant to vancomycin.Staphylococcus spp. showed high resistance to the group of penicillins, I-II generation cephalosporins and some representatives of the III generation. showed good sensitivity to glycopeptides (vancomycin) and linezolid. Streptococcus spp. and Streptococcus pneumonia was resistant to most antibiotic groups. Enterococcus spp. was resistant to all antibiotics except linezolid and vancomycin.An analysis of the sensitivity of Candida fungi to antimycotics showed: nitroxoline - 11.4% of resistant strains, amphotericin and fluconazole - 48.5% of resistant strains, terbinafine - 51.4% of resistant strains.Clinical and biochemical blood tests were performed on an automatic apparatus. The study of the general clinical blood test was carried out on an automatic apparatus Mindray (China) according to the current standards. Venous blood was taken for analysis from the cubital vein several times: in the first hours after the patient was admitted to the hospital, in dynamics - if necessary.Dynamic microbiological control was carried out every 3 days, and, if necessary, in all groups.All patients underwent PCR diagnostics, which helped to promptly diagnose virus-associated pneumonia.Thus, it was revealed that in group I of the studied patients, monitoring of pathogens showed the frequency of the groups Candida, Klebsiella spp., Streptococcus spp., Staphylococcus spp. and Staphylococcus aureus. This group represented 34% of the total sowing rate. Antibiotic therapy was prescribed in accordance with international protocols of empiric antibiotic therapy, and subsequently adjusted in accordance with the results of the antibiogram of the isolated pathogen. Also, when selecting antibiotic therapy, the level of C-reactive protein (CRP) and procalcitonin (PCT) was considered. In group II, the isolation of pathogens was 58% of the total sowing rate. The most frequent and formidable pathogens of this group were Klebsiella pneumoniae - 49.0% within this group, the Candida group - 39.0%, Klebsiella spp. - 19.0%, Staphylococcus aureus - 11.3%, Escherichia coli - 10.4%, Pseudomonas aeruginosa - 8.8%. Such a spectrum of isolated pathogens indicates an unfavorable etiological structure of severe pneumonia. The levels of CRP and PCT in this group were high upon admission to the hospital, and averaged CRP - 73-128 mg / l, PCT - 2.0-4.0 ng / ml and more.The choice of antibiotics and the method of their administration was based on the severity of the patient's condition, analysis of risk factors for encountering resistant microorganisms (the presence of concomitant diseases, previous antibiotic intake, relocation from another hospital, etc.), the results of microbiological diagnostics and other diagnostic methods (CRP and PCT and etc.) (Table 2).Table 2. Laboratory indicators of patients with COVID-19 pneumonia in the ICU
 |
| |
|
Group III consisted of extremely severe patients, the spectrum of isolated microorganisms was 73.6%, Klebsiella pneumoniae, 39.6% of the Candida group, 26.4% Klebsiella spp., 11.3% Escherichia coli, 5.6% Pseudomonas aeruginosa, Enterobacter spp. - 3.8%, Staphylococcus aureus - 1.3%.The quantitative levels of CRP indicators were 90-130 mg / l, PCT - 4.0 ng / ml and higher. Empiric antibiotic therapy was prescribed from the group of "reserve" antibiotics - carbapenems in combination with an inhibitor of protected cephalosporins or piperacillin / tazobactam and / or linezolid or vancomycin, and antifungal drugs were prescribed considering the high release of the Candida group initially.In groups II and III, pathogens were isolated in 27% of cases in the association: Klebsiella pneumoniae + group R. Candida, Klebsiella pneumoniae + Staphylococcus aureus, Klebsiella spp. + Escherichia coli, or Pseudomonas aeruginosa + Staphylococcus spp., Escherichia coli + Candida group + Enterobacter spp. and etc.The spectrum of microorganisms in patients of groups II and III required more intensive antibiotic therapy, considering the existing risk factors (pulse therapy with GCS, long-term therapy with systemic GCS, severe concomitant diseases, ineffective use of systemic antibiotics in previous hospitals, postoperative patients, etc.).Thus, the frequency of isolation of aggressive pathogens, isolation of microbial associations in a third of cases requires prescribing at the first stage of therapy to patients with severe viral-bacterial or secondary pneumonia (more than 70% of lung tissue damage, high levels of CRP and PCT, the presence of concomitant diseases, etc.) antibiotics active against Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and antimycotics in the Candida group. Even with early and adequate antibiotic therapy, mortality in these forms of pneumonia reaches 50%.Empiric antibiotic therapy was prescribed to all groups of patients in accordance with international protocols for anti-infectious chemotherapy and WHO recommendations, as well as on the basis of the results of many years (more than 12 years) research on infection control at the Vakhidov Republican Scientific and Practical Medical Center of Chemistry.Another important result of our study is the description of the features of the clinical, laboratory, and pathogenetic picture of the disease in patients with viral-bacterial and secondary bacterial pneumonia. The results of the study emphasize the importance and dictate the need for a thorough etiological decoding of each case of these pneumonias, which will ensure optimal, etiotropic and pathogenetic therapy, which is reflected in the developed algorithm of antibacterial therapy.Antibiotic therapy should be started empirically within the first 4 hours of admission to the hospital. Depending on the severity of the patient's condition and additional diagnostic methods, modern antibiotics with a wide spectrum of action are prescribed, correction is carried out for the next 48-72 hours based on the results of bacteriological culture of biomaterial from the patient and / or methods for monitoring the effectiveness of antibiotic therapy (possibly with negative results, for example).Also, every 3-4 days a patient is in the ICU, it is necessary to conduct microbiological studies to identify a possible change in the pathogen in order to provide adequate antibiotic therapy timely. In cases of severe (secondary) pneumonia, it is recommended to use original antibacterial drugs with proven activity and effectiveness, since practical experience shows that the use of ineffective generics can lead to the progression of the underlying disease or its prolonged treatment.Activation by the virus of the entire proteolysis system and damage to capillary endothelial cells leads to increased vascular permeability, manifested by an increase in vascular permeability, fragility of their walls, and impaired microcirculation. The virus, entering the bloodstream, also causes suppression of hematopoiesis and the immune system. Secondary immunodeficiency develops, as a result of which various bacterial complications develop. The influenza virus, multiplying in the respiratory tract, causes metaplasia of the ciliated epithelium, the physiological function of which is to cleanse the respiratory tract of dust, bacteria, etc. If the ciliated epithelium is destroyed, it can no longer fully perform its protective functions, and bacteria more easily penetrate into the lungs. Thus, there is a risk of developing bacterial superinfection.
5. Conclusions
An algorithm for antibiotic therapy has been developed, considering the etiological decoding of each case of COVID-19 pneumonia, which allows for optimal, etiotropic and pathogenetic therapy. A comprehensive assessment of the results of bacteriological studies conducted in a multidisciplinary specialized clinic during the period of COVID-19 outbreaks showed an increase in the detection rate of infection with aggressive pathogens and microbial associations of Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus and the advisability of prescribing broad-spectrum antibiotics at the first stage of therapy in intensive care units. The high frequency of isolation of fungi of the genus Candida requires the use of antimycotics from the first hours of admission to the ICU.
References
| [1] | Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020. https://doi.org/10.1001/jama.2020.4031. |
| [2] | Goh KJ, Wong, J., Tien, JC. et al. Preparing your intensive care unit for the COVID-19 pandemic: practical considerations and strategies. Crit Care 24, 215 (2020). https://doi.org/10.1186/s13054-020-02916-4. |
| [3] | Coronavirus: how contact tracers track down the people at risk of infection. In The strait’s times. 2020. Available: http://33h.co/9nq6a. [cited 15 Mar 2020]. |
| [4] | Franki R. Survey: Hydroxychloroquine Use Fairly Common in COVID-19. http://33h.co/9nq62. |
| [5] | K. alilAC, Thomas PG.. Influenza virus-related critical illness: pathophysiology and epidemiology. Crit Care 2019; 23: 258. |
| [6] | Kim D, Quinn J, Pinsky B. et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020; 323: 2085-6. |
| [7] | Maves RC, Jamros CM, Smith AG. Intensive care unit preparedness during pandemics and other biological threats. Crit Care Clin. 2019: 609 - 18. https://doi.org/10.1016/j.ccc.2019.06.001. |
| [8] | Cheng VCC, Chan JFW, KKW T, Yuen KY. Clinical management and infection control of SARS: lessons learned. Antivir Res. 2013; 100: 407 - 19. |
| [9] | WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. |
| [10] | Zhou Y, Qin Y, Lu Y, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J (Engl). March 2020. |
| [11] | Resolution of the President of the Republic of Uzbekistan No. PP-4790 of July 27, 2020 "On measures to organize the activities of the service of sanitary and epidemiological welfare and public health of the Republic of Uzbekistan". |
| [12] | Resolution of the Cabinet of Ministers of the Republic of Uzbekistan No. 446 of July 20, 2020 "On measures for wide coverage of the population by the ambulance service during the fight against coronavirus infection." |
| [13] | Draft clinical guidelines "Community-acquired pneumonia". Russian Respiratory Society, Interregional Association for Clinical Microbiology and Antimicrobial Therapy. 2018 98s. |
| [14] | Internet source https://www.worldometers.info/coronavirus/. |
| [15] | Internet source https://www.vedomosti.ru/society/articles/2020/05/19/830604-rossiya-vihodit. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
