-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(10): 699-704
doi:10.5923/j.ajmms.20211110.07
Received: Sep. 29, 2021; Accepted: Oct. 19, 2021; Published: Oct. 30, 2021

The Features of Microsurgery of Cerebral Arteriovenous Malformation
Khadjibaev A. M., Makhkamov K. E., Makhkamov M. K.
Republican Research Center of Emergency Medicine (RRCEM), Tashkent, Uzbekistan
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim. Description of the features of the microsurgical technique of resection of cerebral arteriovenous malformation (AVM). Material and methods. A retrospective analysis of 26 patients with cerebral AVM was carried out. Of these, 15 patients were females and 11 males, aged 5 to 46 years. All patients underwent neurological examination according to generally accepted standards. After studying the history and physical examination, all patients underwent computed tomography (CT), magnetic resonance imaging (MRI), CT-angiography, and digital subtraction angiography (DSA). The microsurgical technique of access, isolation and microsurgical AVM resection is described in detail. Results. The overwhelming majority of patients with AVM (61.5%) were admitted to a rupture clinic. Of these, with intracerebral hemorrhage (ICH) accounted for 56.3% of cases and 43.7% of cases with subarachnoid hemorrhage (SAH). It was noted that microsurgical resection of complicated AVMs with low grades (grades I and II) is the method of choice. For AVMs with grade III and more methods of choice is a combined treatment method. Conclusion. Increased resectability without imploring postoperative AVM results depends on factors such as a detailed understanding of the AVM anatomical structure and presentation in three-dimensional space, as well as correct exclusion of the adductor vessels and subpial dissection with a combination of endovascular embolization followed by surgical resection.
Keywords: Microsurgical resection, 3D reconstruction, Endovascular embolization, Combined technique
Cite this paper: Khadjibaev A. M., Makhkamov K. E., Makhkamov M. K., The Features of Microsurgery of Cerebral Arteriovenous Malformation, American Journal of Medicine and Medical Sciences, Vol. 11 No. 10, 2021, pp. 699-704. doi: 10.5923/j.ajmms.20211110.07.
Article Outline
1. Introduction
- The incidence of intracranial arteriovenous malformations (AVM) is about 1 per 100,000 per year, in people of working age it is 18 per 100,000. AVMs are characterized by significant morbidity and mortality, with a risk of rupture of about 3% per year [1]. The mortality rate due to AVM rupture is 30%, of which half of the patients have moderate or deep disabling [2]. The high medical and social significance of this disease is determined not only by its prevalence, but also by significant economic damage due to the high frequency of occurrence in people of working age from 20 to 50 years old [3]. Despite the development of endovascular methods of AVM treatment, microsurgical resection is the gold standard. However, to this day there is no consensus on the optimality of the type, stages and timing of surgical treatment, which determines the relevance and disputability of AVM surgery in modern neurosurgery.
2. The Aim of the Study
- The aim of our study was to describe the features of the microsurgical technique of AVM of cerebral vessels.
3. Material and Methods
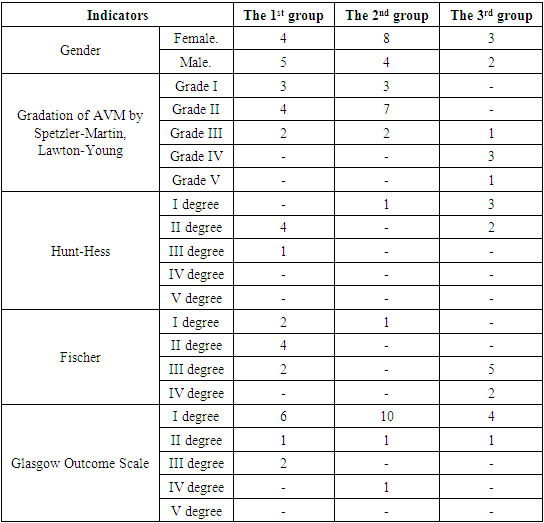
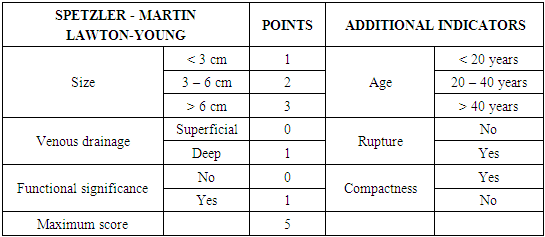
- We performed a retrospective analysis of 26 patients with cerebral AVM. (Table 1). 15 patients were females and 11 males, aged from 5 to 46 years. All patients underwent neurological examination according to generally accepted standards. After studying the history and physical examination, all patients underwent CT, MRI, CT-angiography, and DSA. The application of these research methods is justified by the fact that they are all interconnected. With the help of CT, we evaluated the signs of rupture, the presence of hemorrhage and the volume of hematomas, as well as the presence of calcified areas. MRI was performed to assess the compactness of the AVM. CT angiography and DSA were used to visualize not only the nucleus itself - the "nidus" of the AVM, but also the supplying vessels and the participation of the blood supplying arterial pools, the size and course of the vessels, as well as the points of connection with the "nidus", the nature of the draining veins, their size and flow blood in the draining vein, in particular, inside the "nidus" (Fig. 1). The relationship of AVM with surrounding anatomical structures was also studied, the presence of thrombosis of the AVM was determined, which is extremely important for planning surgery. In addition, 3D modeling of intracerebral vessels was of decisive importance in preoperative planning, which made it possible to visualize the relationship of microanatomical structures, i.e. pathological vascular plexus in three-dimensional space. For the purpose of a more detailed study of the results of surgical treatment of AVMs, a modified Spetzler-Martin & Lawton-Young classification was used [4] (Table 2).
|
|
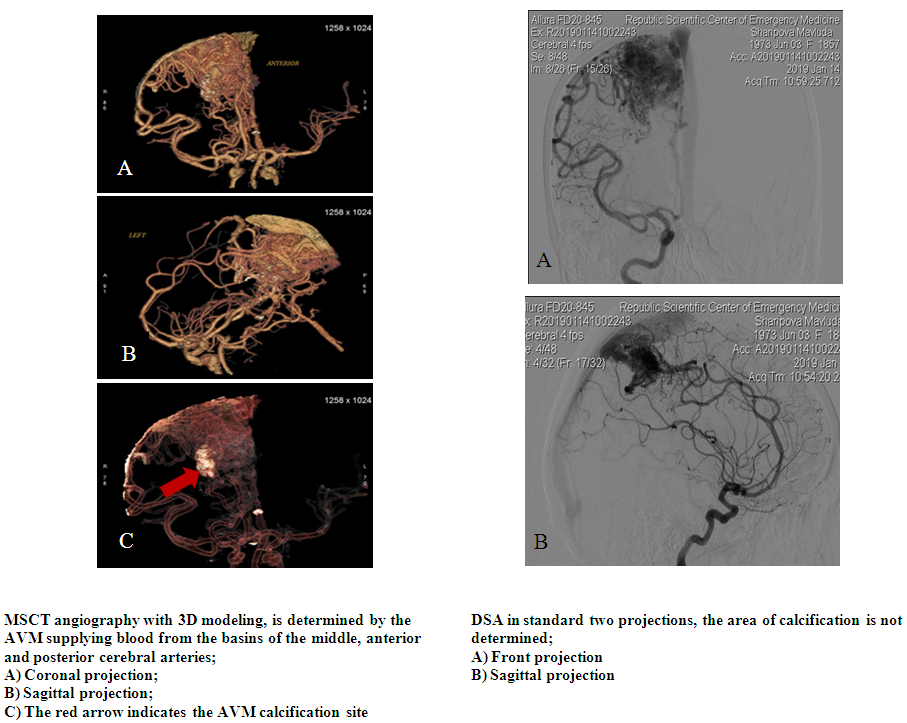 | Figure 1 |
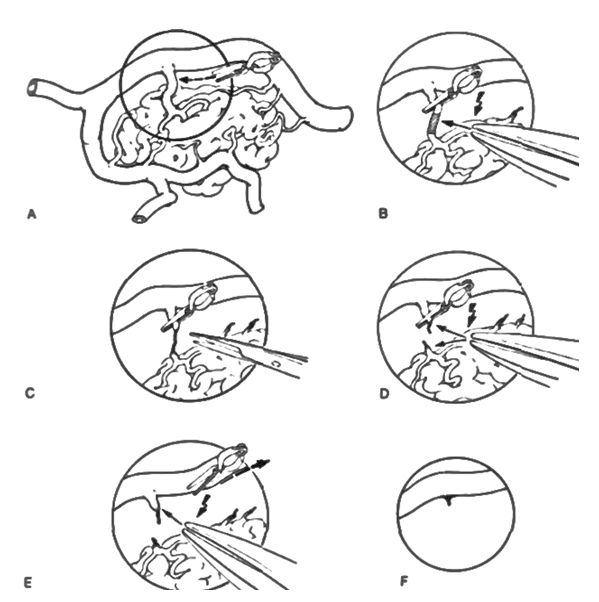 | Figure 2. Schematic representation of the AVM devascularization stage |
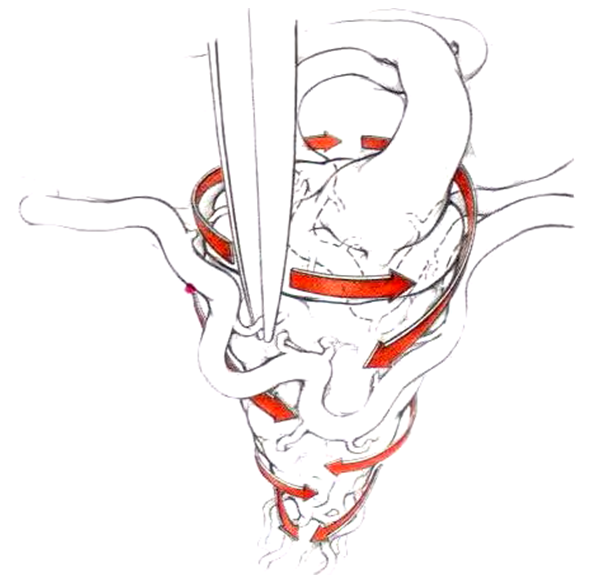 | Figure 3. Schematic representation of the AVM devascularization technique |
4. Results
- The results of our study showed that microsurgical interventions were performed in 34.6% (n = 9) cases, 46.2% (n = 12) endovascular (embolization) interventions and in 19.2% (n = 5) cases, there was a combination of partial embolization followed by microsurgical resection. Our study was observed in 61.5% (n = 16) cases with ruptured AVM’s. From them in 56.3% of these, (n = 9) cases - intracerebral hemorrhage (ICH) and in the remaining 43.7% (n = 7) cases, SAH. Among ICH, in 33.4% (n = 3) cases, the hematoma was located in the frontal lobe, in 11.1% (n = 1) cases, in the temporal lobe, in 22.2% (n = 2) cases, in the parietal lobe with spread to the mediobasal regions and the ventricular system, in 22.2% (n = 2) cases in the occipital lobe and in 11.1% (n = 1) was subtentorial hemorrhage. Of the patients who underwent a rupture, 25% (n = 4) cases were admitted in the acute period, in 43.7% (n = 7) cases in the subacute period and in 31.3% (n = 5) cases in the cold period. In the remaining 38.5% (n = 10) cases, the clinical picture was presented by a pseudotumorous course (general cerebral symptoms, convulsions and focal deficit). From 34.6% (n = 9) cases, in the 22.2% (n = 2) showed signs of partial thrombosis of the malformation after the previous hemorrhage. Considering these signs of a partially thrombosed node, it was decided to limit ourselves to microsurgical resection. In one case, there was a postoperative complication in the form of hemorrhage in the bed of the resected malformation with the emergence of persistent neurological deficit in the form of deep hemiparesis, and in another case, in one patient, elements of motor aphasia. 19.2% (n = 5) of patients with grades III and IV, one patient in the postoperative period had a slight neurological deficit in the form of foot monoparesis, which regressed within 10 days.
5. Discussion
- Surgical treatment of AVMs should be adapted depending on the AVMs’ gradation. Despite the fact that surgical resection is the gold standard, with the development of minimally invasive interventions, the use of microsurgery has become more rational for small (I and II grades), convexitally located AVMs. For AVM grade III and more, one-stage total embolization is, in most cases, hardly possible. Partially embolized AVMs of grade III and higher in the early postoperative period may be complicated by rupture and ICH from the residual part of the AVM. To date, the most optimal choice of AVM treatment method is a combination of partial endovascular embolization and microsurgical resection [7]. The incidence of vascular catastrophes due to AVM rupture is 1-2% among all strokes, in 4% of cases accompanied by ICH and in 9% of cases with SAH [2]. In our results the incidence of ICH was 61.5%.In the studies of carried out by Hamilton M.G. and Spetzler R.F, it was noted that the risk of developing neurological deficits and deaths in the postoperative period after microsurgical resection for AVMs of I and II grades is 1%, 3% for AVMs of grades I and II, 31% and 50% - for AVMs IV and V grades, respectively [8,9]. The presence of hemorrhage or a history of early hemorrhage is a criterion for active surgical tactics. However, there are strict indications of the patient selection criterion for microsurgical resection, such as the age and general condition of the patient, AVM size, localization, angioarchitectonics of malformation, surgical accessibility, and cerebral circulation [10]. Acute hemorrhage has a number of advantages and disadvantages in AVM surgery. The advantage is that the hematoma envelops the malformation around its perimeter and thus subpial dissection is achieved. Also, as the hematoma evacuates, a residual space and a corridor for manipulation appears, minimizing trauma to the already injured medulla. Despite this, one of the most negative effects of acute hemorrhage is a significant decrease in the visualization of the AVM “nidus” due to the staining of the imbibed medulla and the adductor and outlet vessels [8,9,11,12]. Analysis of the literature also showed that the risk of hematoma in the bed in the postoperative period is about 40% [4], which was 11.1% in our research.Despite the fact that microsurgical resection is the most radical method of treatment, the majority of patients (42.5%) require a combined method of treatment, i.e. the combination of microsurgical resection and preliminary preoperative embolization [13]. (Fig. 4).
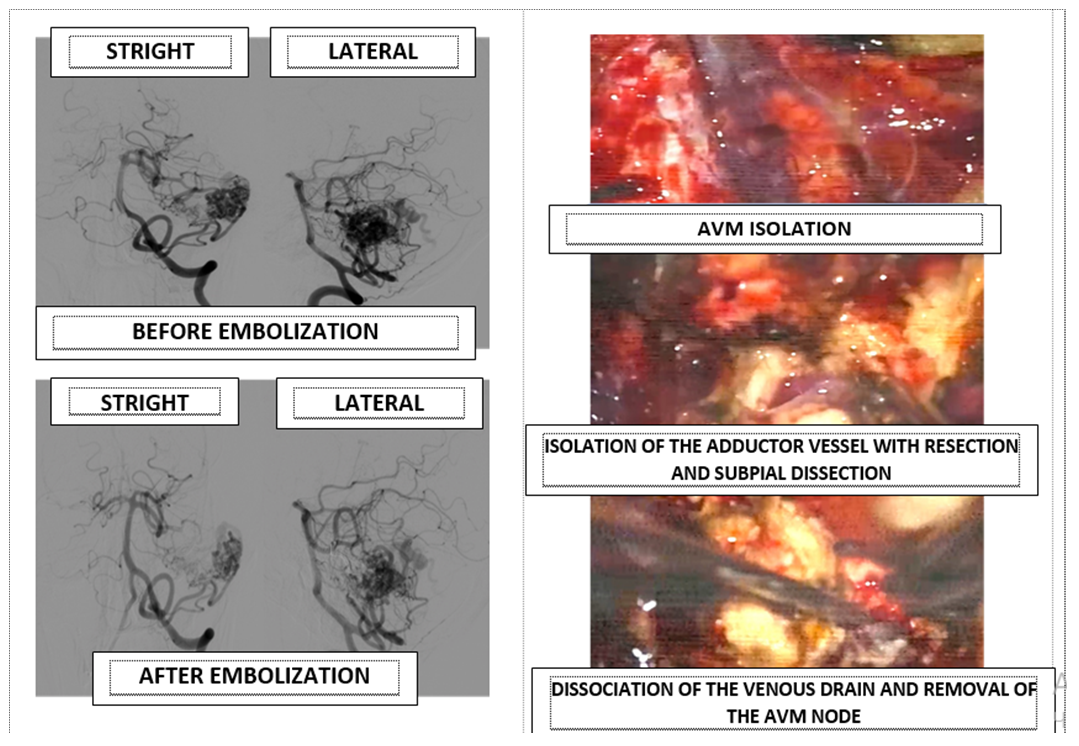 | Figure 4. Combined technique of AVM treatment, endovascular embolization of the adductor vessels and partly of the AVM “nidus” followed by microsurgical resection |
6. Conclusions
- Thus, a detailed study of the anatomical structure of the AVM, and representation in three-dimensional space using three-dimensional reconstructive anatomy, the correct choice of craniotomy and angle of attack depending on the localization of the AVM, as well as the correct shutdown of the adductor vessels and subpial dissection, as well as a combination of endovascular embolization with subsequent surgical resection, which allows not only to increase the resectability of the AVM, to minimize the trauma of the medulla and surrounding vessels in the surgical corridor, without begging for postoperative results of surgery.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML