-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(10): 673-677
doi:10.5923/j.ajmms.20211110.03
Received: Sep. 25, 2021; Accepted: Oct. 14, 2021; Published: Oct. 20, 2021

Changes in Morphometric Parameters of the Lymphoid Tissue of the Small Intestine in the Conditions of Polypragmasia
Nasirova Sabina
Bukhara State Medical Institute, The Republic of Uzbekistan
Correspondence to: Nasirova Sabina, Bukhara State Medical Institute, The Republic of Uzbekistan.
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Various clinical symptoms of inflammatory diseases in combination with the limited possibilities of etiotropic therapy, a large number of symptomatic agents became the causes of polypharmacy. Unreasonable treatment often slows down the natural healing process. Recently, the gastrointestinal tract is considered as an essential component of the general defense of the body. Therefore, without accurate knowledge of the structural features and the effect of drugs on the morphological structure of the small intestine, it is impossible to understand the degree of their participation in the body's defense reactions.
Keywords: Morphology, Small intestine, Polypharmacy
Cite this paper: Nasirova Sabina, Changes in Morphometric Parameters of the Lymphoid Tissue of the Small Intestine in the Conditions of Polypragmasia, American Journal of Medicine and Medical Sciences, Vol. 11 No. 10, 2021, pp. 673-677. doi: 10.5923/j.ajmms.20211110.03.
Article Outline
1. Introduction
- The protective function of the intestine is the main barrier to foreign microorganisms. One of the large peripheral parts of the immune system is the gut-associated lymphoid tissue. The immune structures associated with the mucous membrane are immunologically active tissue. About 80% of all immune competent cells in the body are associated with the intestinal mucosa. The integrity of the mucous membrane is one of the main qualities of a healthy organism. Among the immune formations of the digestive system, lymphoid nodules (Peyer's patches) of the small intestine play an exceptional role. They, like the thymus, amygdala, mammalian appendix, belong to the lymphoepithelial organs, in which lymphopoiesis occurs and are in close interaction with the reticular tissue, the epithelium. The use of a large number of drugs can adversely affect the function and structure of the mucous membrane of the small intestine. Anti-inflammatory drugs are one of the most commonly used drug groups in medicine. Identifying the side effects of polypharmacy is an urgent problem today. The aim of this study was to study polypharmacy with anti-inflammatory drugs on the structural and cellular structure of the wall and lymphoid nodules of the small intestine of white rats.
2. Material and Research Methods
- Sexually mature white outbred rats weighing 200-250 g, 5 months of age, were used in the work. The experiment was carried out on 120 animals kept in the general regime of the vivarium. For 10 days, the animals were injected enterally through a tube into the stomach with anti-inflammatory drugs at the rate of hydroxychloroquine 6.5 mg/kg, paracetamol 15 mg/kg, aspirin 5 mg/kg, dexamethasone 0.1 mg/kg. All laboratory animals were divided into 4 groups: group 1 - control animals (30 rats) receiving distilled water through a tube, group 2 (30 rats) - laboratory animals that received 2 types of anti-inflammatory drugs (Hydroxychloroquine, dexamethasone); Group 3 (30 rats) - laboratory animals that received 3 types of anti-inflammatory drugs (Hydroxychloroquine, dexamethasone, paracetamol); Group 4 (30 rats) - laboratory animals that received 4 types of anti-inflammatory drugs (Hydroxychloroquine, dexamethasone, paracetamol, aspirin). The material was collected on day 11, after 10 days of drug administration. The animals were slaughtered at the appropriate time in the morning, on an empty stomach by means of instant decapitation under ether anesthesia. After opening the abdominal cavity, the small intestine was removed. To conduct a morphological study, the mesentery of the small intestine was mobilized (along the intended line of intersection of the intestine), followed by its resection and dissection along the mesenteric edge along the entire length with microscissors. After that, the length of the mesenteric part of the small intestine, the width of the small intestine in the initial, middle and final parts of the mesenteric part of the small intestine were measured with a millimeter ruler. After washing the obtained total preparations of the small intestine in running water, they were stained with hematoxylin - H (Harris) and clarified with 3% acetic acid, followed by repeated washing of the preparations in distilled water. The number, shape, size of aggregated lymphoid nodules, the distance between aggregated lymphoid nodules, the number of lymphoid nodules in them, and the area of the mesenteric part of the intestine covered with aggregated lymphoid nodules were studied on total macropreps stained with Harris's hematoxylin. Shape, size of lymphoid nodules in aggregated lymphoid nodes, distance between nodules. The number of single lymphoid nodules in 1 cm2 of the mesenteric area of the small intestine. For the morphological and morphometric study of the mesenteric part of the small intestine and its lymphoid formations, pieces were taken from the initial, middle and final parts of the mesenteric part of the small intestine. Pieces of tissue were fixed in a 10% solution of neutral formalin. After appropriate processing, the material was embedded in paraffin and sections with a thickness of 4-6 mm. were prepared, which were stained with hematoxylin and eosin. Sections were examined morphometrically, using an eyepiece micrometer DN-107T/ Model NLCD-307B (Novel, China), the thickness of the mucosa, submucosa, muscle and serous membranes of the mesenteric part of the small intestine was measured, especially the places where the lymphoid formations are located.
3. Results and Discussion
- Microscopic examination of the middle part of the small intestine of white rats of the control group showed that the mucous, muscular and serous membranes are well developed and have distinct boundaries. The mucous membrane contains villi of large diameter and crypts of smaller diameter. On the surface of the villi and crypts are cylindrical epithelial cells with oval nuclei. Among the epithelial cells are goblet cells, the largest number of which is observed in crypts. According to our results, the study of the middle part of the wall of the small intestine, there is a different degree of morphological changes when exposed to different amounts of drugs. It was found that after exposure to drugs, the size and ratio of structural components in the walls of the small intestine change markedly. In the mucous membrane of the small intestine of the experimental group of animals, an intense desquamation of epithelial cells into the intestinal lumen was observed at the tops of the villi. The stroma of the villi is edematous, it contains a large number of macrophages and lymphocytes, and necrosis of the villi is observed in some areas. Figure 1.
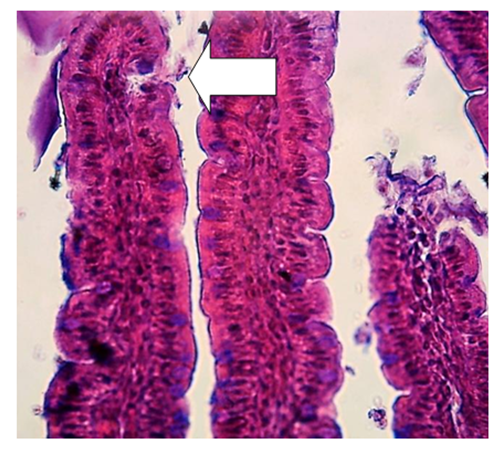 | Figure 1. Intestinal villus. Arrows show the areas of desquamation of epithelial cells of the villi of white rats. Hematoxylin-eosin staining |
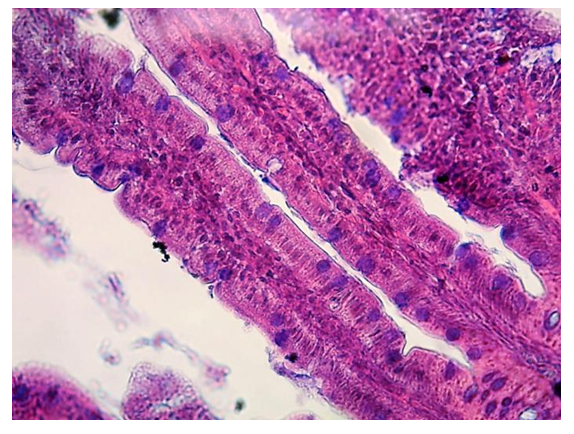 | Figure 2. Intestinal villus. Arrows indicate goblet cells filled with mucus. Hematoxylin-eosin staining |
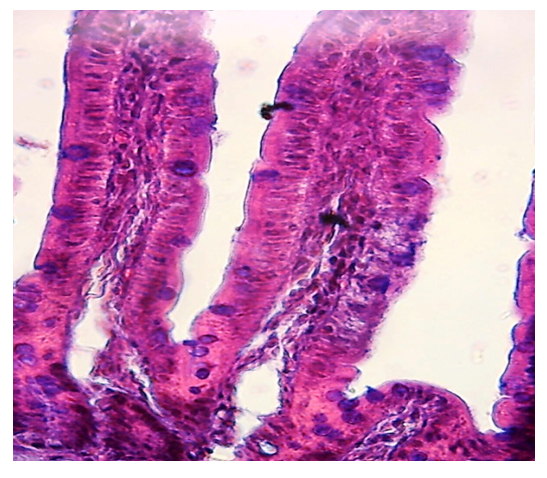 | Figure 3. Intestinal villus. Heteromorphic structure. Edema and atrophy of epithelial cells of villi of white rats, desquamation of epithelial cells. Hematoxylin-eosin staining |
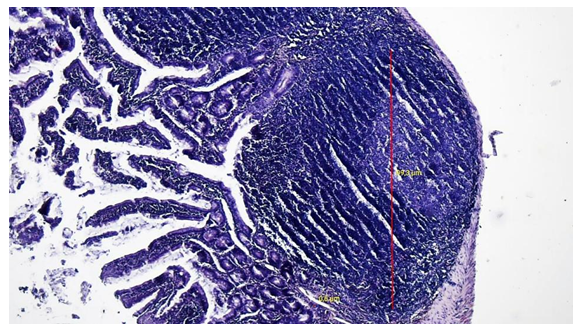 | Figure 4. Germinative zone of the Peyer's patch lymph node in the ileum of 5-month-old rats of the control group. Coloring: hematoxylin-eosin |
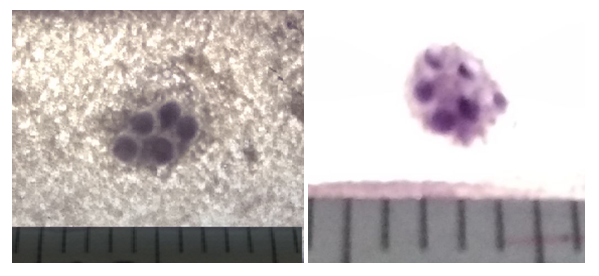 | Figure 5. Aggregated oval-shaped lymphoid nodule in the proximal part of the small intestine of a 5-month-old rat of the control and fourth groups. Coloring according to Helman |
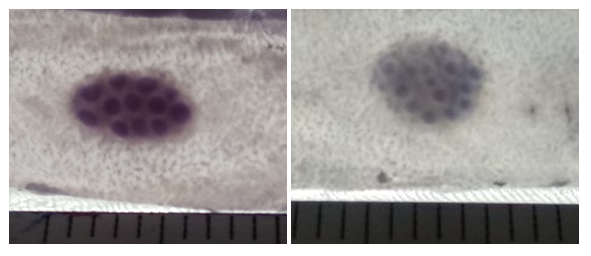 | Figure 6. Aggregated oval-shaped lymphoid nodule in the middle part of the small intestine of a 5-month-old rat of the control and fourth groups. Coloring according to Helman |
4. Сonclusions
- As a result of studies in the wall of the small intestine of rats treated with anti-inflammatory drugs, changes of a destructive nature were found, depending on the use of the amount of drugs. Destructive changes are characterized by edema of the stroma of the villi, their desquamation into the intestinal lumen, a decrease in the height of the villi, which were clearly observed in the fourth group when using four types of anti-inflammatory drugs. At the same time, the observed increase in the number of goblet cells in the villi. Presumably, this is due to an increase in degenerative processes in epithelial cells under the influence of polypharmacy. Polypharmacy also negatively affected the number and size of single lymph nodes. Lymphoid nodules react to polypharmacy with anti-inflammatory drugs with a decrease in size. The size of group lymph nodes and the size of lymphoid nodules in them against the background of polypharmacy decreases, which depends on the dose and time of use. It was found that with polypharmacy, the distance between the group lymphoid nodes decreases due to a decrease in size. In polypharmacy, the area of the small intestine covered with lymphoid nodules decreases, in contrast to the control group, which indicates a decrease in the functional activity of the intestinal lymphoid tissue after exposure to four types of anti-inflammatory drugs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML