-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(9): 658-661
doi:10.5923/j.ajmms.20211109.11
Received: Aug. 31, 2021; Accepted: Sep. 19, 2021; Published: Sep. 26, 2021

Morphological Aspects of Intestinal Insufficiency in Experimental Peritonitis
Dilorom Bakhtiyarovna Adilbekova1, Sayfiddin Risbaevich Baymakov2, Khusain Salimatdinovich Abdurazzakov3
1Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
2Department of Surgery, Tashkent State Dental Institute, Tashkent, Republic of Uzbekistan
3Department of Surgery, Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
Correspondence to: Dilorom Bakhtiyarovna Adilbekova, Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Republic of Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Our studies show that pathomorphological changes in the small intestine in acute experimental peritonitis are characterized by dystrophic, inflammatory-destructive and vascular disorders in the vascular-tissue structures of the small intestine wall. In the pathogenesis of damage to the small intestine, deep disorders in the vessels of the microhemocirculatory course from hyperemia to inflammatory and destructive changes are of great importance, subsequently leading to a violation of the trophic cells, tissue hypoxia, damage to cellular elements, violation of cellular metabolism, deficiency in energy and plastic materials, accumulation of perverted metabolic products in cells and tissues.
Keywords: Acute experimental peritonitis, Small intestine, Vessels, Tissue structures
Cite this paper: Dilorom Bakhtiyarovna Adilbekova, Sayfiddin Risbaevich Baymakov, Khusain Salimatdinovich Abdurazzakov, Morphological Aspects of Intestinal Insufficiency in Experimental Peritonitis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 9, 2021, pp. 658-661. doi: 10.5923/j.ajmms.20211109.11.
Article Outline
1. Introduction
- Intestinal insufficiency, occupies a leading place among all complications of acute pathology of the abdominal cavity, the mortality rate is high (from 15% to 40%) and has no tendency to decrease [1,6,11]. Taking into account the fact that the intestines are the main source of intoxication, the intestinal insufficiency syndrome is currently considered as a disease of the entire digestive tract with the appearance of multiple organ changes [3,8,10]. The severity, prevalence, severity and phases of pathological processes in the abdominal cavity determine the degree of morphofunctional changes in the intestinal wall. In the literature available to us, we noted the lack of data on the degree of pathomorphological changes in the intestine in acute diseases of the abdominal cavity. In this regard, the study of this issue is an urgent problem for both clinical and fundamental medicine.The aim of the study was to study and evaluate morphological changes in the intestinal wall in acute experimental peritonitis.
2. Materials and Methods of Research
- The object of the study is the materials of the small intestine of rats removed on the 1st, 2nd, 3rd and 4th days of the experimental model of peritonitis (the experimental group was 50 rats). Peritonitis was caused by the introduction of a filtered 2% autofecal suspension in the amount of 350 mg/kg into the abdominal cavity, no later than 20 minutes after preparation under ether anesthesia. The control group consisted of 40 rats, normal (physiological) saline in an equivalent volume was injected into the abdominal cavity them. In order to avoid damage to internal organs when fecal suspension was introduced into the abdominal cavity, the animals were placed vertically, with the caudal end up. To achieve the goal and set tasks, general morphological, electron microscopic research methods were used. All studies were conducted in accordance with the “International Recommendations for conducting biomedical research using Animals” (1985, 1989). The morphological data obtained during the study were subjected to statistical processing on a Pentium-IV personal computer using the Microsoft Office Excel-2012 software package, including the use of built-in statistical processing functions. The differences satisfying P<0.05 were considered reliable.
3. Results
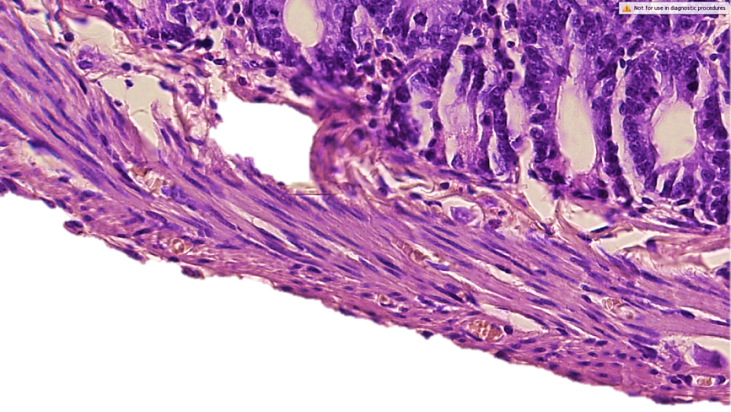
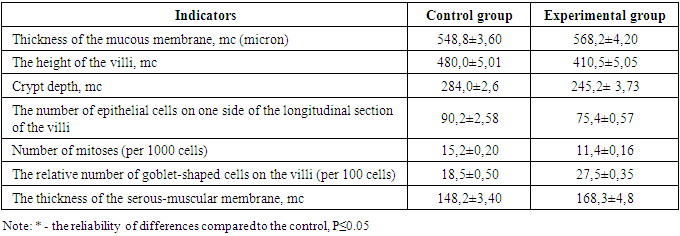
- 1-2 hours after the simulation, the first clinical signs of peritonitis were observed in the experimental rats: restless animals, refusal of food, tension in the abdominal area. After 6-12 hours, the animals are sedentary, inhibited, grouped in the corner of the cage, sluggish, apathetic to food, there is frequent shallow breathing, ruffled hair. After a day, the abdomen is sharply swollen, with laparotomy, the accumulation of 1.5-2 ml of serous-hemorrhagic fluid in all parts of the abdomen was determined. The leaves of the peritoneum and mesentery of the small intestine were dull, rough, there was vasodilation, small-point hemorrhages and inhibition of intestinal motility. Morphological examination of the wall of the small intestine revealed that the intestinal wall is edematous, loosened, infiltrated. There is edema in the mucous membrane, infiltration by mononuclear cells. Moderate dystrophic changes were detected in the epithelial cells of the mucous membrane. The stroma of the villi and crypts is edematous, infiltrated, loosened. In the tops of the villi (strands), desquamation of the epithelial layer is determined, which led to the formation of microerosion. The height of the villi and crypts, the total number of epithelial cells and the number of mitotically dividing cells are reduced compared to the indicators of control animals. There is an increase in the proportion of swollen goblet cells (see Table 1). Electron microscopically, fine-grained granules and transparent vacuoles are detected in the cytoplasm of enterocytes, some cells are enlarged in size, are in a state of pronounced cytoplasmic edema with a shift of the nuclei to the apical edge of the cell.In the submucosal layer, there is edema, loosening, infiltration by mononuclear cells. There is pronounced edema in the muscle membrane, infiltration of connective tissue layers by mononuclear cells. Subserous edema, swelling, loosening and subtotal desquamation of mesothelial cells are also detected in the serous membrane of the intestinal wall (Fig. 1).
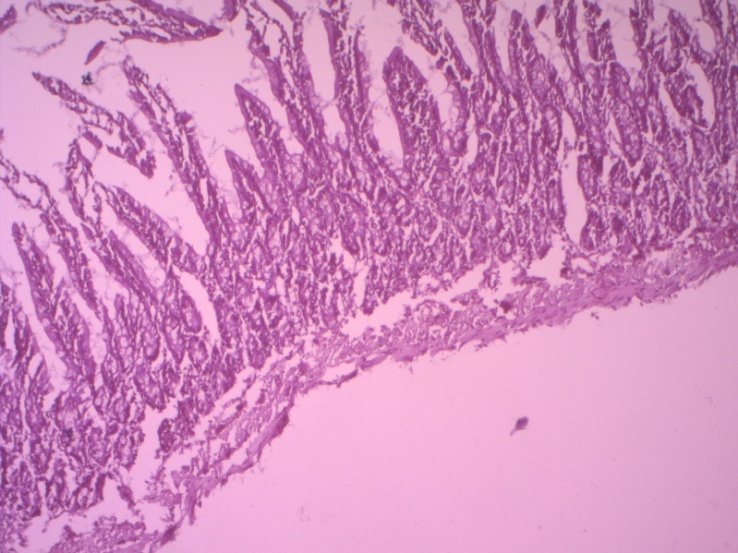 | Figure 2. Morphology of the small intestine wall on day 3 in peritonitis. Destructive changes in all layers of the intestinal wall. Staining with hematoxylin-eosin. 10x10 |
|
|
4. Discussion
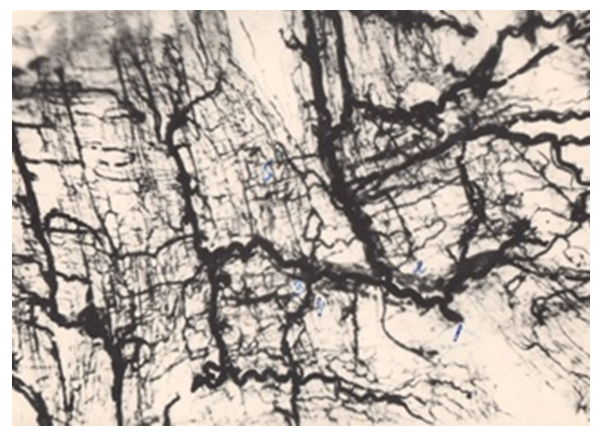
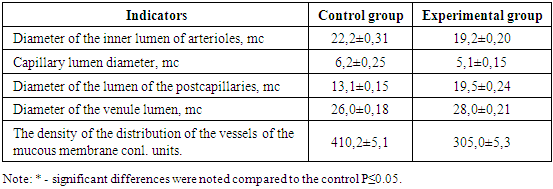
- Pathomorphological changes in the small intestine in acute diffuse peritonitis confirmed the development of intestinal insufficiency syndrome and impaired intestinal function [2,4,5,7,9]. Our scientific studies showed a violation of microcirculation, which subsequently led to an aggravation of dystrophic and necrobiotic disorders in the tissue structures of the small intestine wall. At the beginning of the pathological process, dystrophic changes of various degrees of severity were observed, and then – necrobiotic. More pronounced pathomorphological changes in the vessels were detected at the end of the first day: there was a decrease in the internal diameter of the vessels of the arterial link, swelling and thickening of their walls. These processes gradually progressed on the 2, 3, 4 day of the experiment. In the vessels of the venous department, pronounced fullness, expansion of the internal lumen, microthrombosis, extravasates, a decrease in the density of the distribution of vessels were observed, which led to a violation of the metabolic processes between the blood and the intestinal wall tissue. All these pathomorphological processes in vascular structures negatively affected the state of cellular metabolism and caused dystrophic and destructive changes in the tissue structures of the intestinal wall.
5. Conclusions
- 1. Intestinal insufficiency in experimental peritonitis is characterized by vascular, inflammatory-destructive and dystrophic disorders in the vascular-tissue structures of the small intestine.2. The pathomorphological disorders in peritonitis are based on deep vascular disorders, as evidenced by edema and swelling and high variability of the walls, expansion, fullness of venous vessels, microthrombosis, violation of the permeability of the vessel walls, multiple extravasates, a decrease in the density of the distribution of vessels in the microhemocirculatory flow.3. All these processes were accompanied by a violation of trophic activity in the intestinal wall, tissue hypoxia, damage to cellular elements, violation of cellular metabolism, deficiency in energy and plastic materials, accumulation of perverted metabolic products in cells and tissues.4. A deep understanding of the pathogenesis of morphological disorders of this condition determines in the future the implementation of targeted, scientifically based, highly effective complex therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML