-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(9): 640-644
doi:10.5923/j.ajmms.20211109.08
Received: Aug. 21, 2021; Accepted: Sep. 15, 2021; Published: Sep. 26, 2021

A Comparative Histological Study of Preeclampsia Effects in Pregnancy Period
Shukurov Ural1, Tursunov Khasan1, Murkhamedova Nurkhan2, Dadamukhamedova Khalida1, Yusupova Shakhlo3
1Department of Pathological Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan
2Department of Medical and Biological Chemistry, Tashkent Medical Academy, Tashkent, Uzbekistan
3Republican Scientific and Practical Center of Forensic Medicine, Tashkent, Uzbekistan
Correspondence to: Shukurov Ural, Department of Pathological Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the effects of preeclampsia and to consider a comparative analysis of the structural structure of lymph nodes located at different locations. Materials and methods: Lymph nodes located at different locations of women who died during pregnancy were examined in two groups: control, comparison group - women who died from other diseases and preeclampsia. Results: it was noted that in preeclampsia, the morphometric parameters of the morphofunctional areas of the lymph nodes in all locations were altered. In the paratracheal lymph nodes, the area of lymphoid follicles, paracortex, and pulp ducts is enlarged, and the area occupied by other zones is reduced. A more reliable increase in the area of lymphoid follicles and pulp ducts in the mesentery and subhepatic lymph nodes was observed. Conclusion: we have scientifically evaluated the effect of preeclampsia by a significant enlargement of the paracortex area in the submandibular lymph nodes, almost doubling this group compared to the control group, and a significant decrease in the sinus and cortical area of the cerebral cortex in these lymph nodes.
Keywords: Preeclampsia, Lymph node, Pathomorphology, Pregnancy women, Lymphoid follicle
Cite this paper: Shukurov Ural, Tursunov Khasan, Murkhamedova Nurkhan, Dadamukhamedova Khalida, Yusupova Shakhlo, A Comparative Histological Study of Preeclampsia Effects in Pregnancy Period, American Journal of Medicine and Medical Sciences, Vol. 11 No. 9, 2021, pp. 640-644. doi: 10.5923/j.ajmms.20211109.08.
Article Outline
1. Introduction
- Preeclampsia is one of the most common complications of pregnancy and remains one of the causes of maternal deaths. As a result of the analysis of maternal deaths from severe gestosis, the following patterns were revealed [1]: pathomorphological changes in organs were characterized by pronounced damage to the cell structure due to circulatory disorders, the development of disseminated intravascular coagulation syndrome, microcirculation block and severe ischemia; in all cases of maternal deaths there were combined forms of gestosis, which is confirmed by pathomorphological changes in target organs.According to Russian researchers, the preventability and conditional preventability of maternal mortality from PE, eclampsia in 2017 was 80.9% [2]. They attributed the main complications that caused the death: cerebral edema with dislocation syndrome, multiple organ failure syndrome, massive coagulopathic bleeding. In addition, the authors noted an increase in the incidence of acute cerebrovascular accidents.Preeclampsia is a specific hypertensive disorder associated with pregnancy that is diagnosed by the presence of three main features: hypertension, proteinuria, and edema that manifests after 20 weeks of gestation (usually starts at 32 weeks, but may appear earlier in patients with pre-existing kidney disease, hypertension, etc.) [3]. From a clinical point of view, eclampsia is preeclampsia complicated by convulsions, disseminated intravascular coagulation, damage to the liver, kidneys, brain, heart and placenta due to thrombosis of arterioles and capillaries.Early-onset disease is usually associated with improper implantation, resulting in decreased blood supply to the placenta. Later, the disease is associated with excess or ischemic trophoblast in the maternal circulation. Maternal hypertension due to the release of vasoactive substances by the endothelium that damage the endothelium.The triad of placenta, fetus and mother continues to form a complex functional balance during the prenatal period, and dysfunction of one of them can jeopardize others. Placental dysfunction can often lead to preeclampsia or eclampsia [4]. Preeclampsia and eclampsia remain the leading causes of maternal and perinatal mortality and morbidity worldwide, causing 12–15% of direct maternal deaths [5]. The incidence of preeclampsia is 2–0%, depending on the population studied; and the definition of preeclampsia and age up to 20 years are significantly affected [6]. Although little is known about the pathogenesis of preeclampsia, a recent study found that deficiencies in vitamin C, vitamin E, fat, zinc, and calcium, as well as excess calories and carbohydrates, were associated with an increased risk of preeclampsia and eclampsia [7].Pregnancy is a serious problem for the maternal immune system, which must carry the fetal alloantigen encoded by the paternal genes. In addition to their role in stimulating maternal and fetal immune tolerance, accumulating evidence indicates that decidual immune cells are involved in several processes necessary for a successful pregnancy, including trophoblast invasion and tissue and coiled artery remodeling [8].The placenta is the link between the mother and the developing fetus and plays a central role in a number of fundamental biological processes necessary for the survival of the fetus. Two such processes are immunological tolerance to the fetus and fluid balance between mother and fetus [9]. It is the lymphatic system that is responsible for maintaining hemeostasis of fluid in tissues and regulation of immunological reactions. A limited number of studies have been conducted to investigate the lymphatic vascular system and lymphangiogenesis in the human uterus. There is conflicting evidence about the lymphatic vasculature in the endometrium. So some researchers found lymphatic vessels in the functional zone of the human endometrium in 62% of the samples, while only in the basal region.
2. Aim of the Research
- Morphometric comparative analysis of the structural organization of lymph nodes of different localization with an assessment of the comparative structural coefficients of different zones of the lymph node in preeclampsia.
3. Materials and Methods
- Lymph nodes of different localization of women who died during pregnancy were studied in two groups: the control group, the comparison group - women who died from accidents and the experimental group - women who died from preeclampsia. For histological examination, the objects were visceral - mesenteric, paratracheal, subhepatic and subsplenic lymph nodes of different topographic groups. The lymph nodes were fixed in 10% neutral formalin. This was followed by the classical scheme of wiring and embedding the material in paraffin, followed by the preparation of histological sections. Histological sections were made longitudinally and always strictly through the gates of the lymph nodes, then stained with hematoxylin and eosin, azure and eosin. The morphometric analysis of the structural components of the lymph node was carried out using the Image-Pro Plus 4.1 software. using a morphometric grid [10], which was applied to the lymph node section. The number of mesh crossings for the entire section as a whole and separately for each of the structural components of the lymph node (capsule, cortical plateau, lymphoid nodules, paracortex, pulp cords, sinuses) was calculated with a percentage conversion. To compare the structural organization of lymph nodes of different topographic groups, a methodological technique was applied, which consists in standardizing the total cross-sectional area of the lymph node, when its size is taken as 100%. In this case, it becomes possible to compare the degree of development of structural and functional zones with each other in the lymph nodes of a different topographic group. Some morphofunctional coefficients of the lymph node were calculated, showing activation or inhibition of organ function. The work used a statistical method with the determination of the arithmetic mean, root-mean-square error and significance of differences at p <0.05 using the StatPlus Pro 2009 program.
4. Results
- The study of lymph nodes of different localization of the deceased women in the control group showed that morphometric indicators of various morphological and functional zones differ from each other. The area of the cortical plates of the paratracheal lymph nodes is wider than that of the analogous zone of the lymph nodes of other localizations. Lymphoid follicles of lymph nodes of all types of localization almost occupy the same area and averaged 23.1 ± 2.7%, in the mesenteric lymph nodes this indicator is relatively higher than in other localizations (table 1). The area of the paracortex of the sub-splenic lymph nodes had the largest indicator and averaged 24.6 ± 3.2%, in the lymph nodes of other localizations this indicator was smaller, especially in the mesenteric lymph nodes (16.4 ± 1.4). Lymph nodes in general, in particular, their individual morphofunctional zones with preeclampsia experience both functional and morphological tension. Significant changes in the percentage of all morphofunctional zones of paratracheal, mesenteric, subhepatic and subsplenic lymph nodes are noted.It was noted that with preeclampsia, the morphometric indicators of the morphofunctional zones of the lymph nodes of all localizations underwent changes. In the paratracheal lymph nodes, the area of the lymphoid follicles (Fig. 1), the paracortex and pulp cords expanded, while the occupied area of other zones decreased. In the mesenteric and subhepatic lymph nodes, a more significant increase in the area of lymphoid follicles and pulp cords was observed (Fig. 2) (table 1). In the sub-splenic lymph nodes, a significant expansion of the area of the paracortex was noted and was almost twice as large as in the control of the same group. In these lymph nodes, a significant decrease in the area of the cortex and sinuses of the medullary layer was also noted.
|
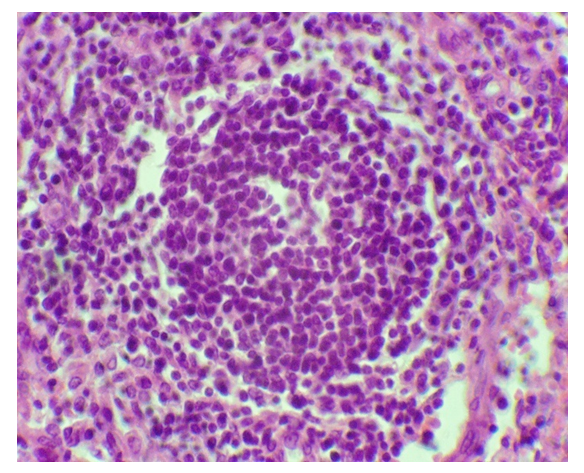 | Figure 1. Expansion of the area of lymphoid follicles with preeclampsia. Paint.: G-E. Uv: 10x40 |
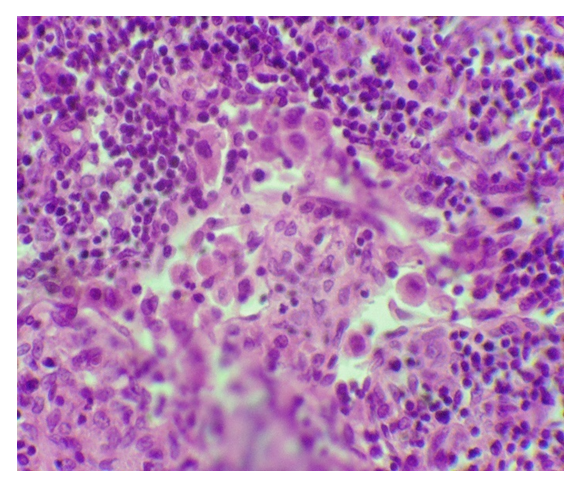 | Figure 2. An increase in the volume of the pulpy cords of the lymph node with preeclampsia. Paint.: G-E. Uv: 10x40 |
5. Discussion
- Revealed in the lymph nodes of different localization of the control group and in preeclampsia, apparently, reflect the stages of "alarming" general adaptation syndrome. This opinion is consistent with the conclusions of the works of Yu.I. Borodin (2011), who believes that one of the first reactions to endointoxication is the migration of lymphoid cells from peripheral to central organs, immunogenesis, which increases the immune competence of the bone marrow and the body's resistance to damaging factors. At the same time, the proportion of the cross-sectional areas of the structural components on the section of paratracheal and mesenteric lymph nodes almost does not differ from the parameters of the control group. A noticeable change in the structure of the parenchyma of the mesenteric groups of lymph nodes is observed due to the cerebral cords, the area of the medulla increases significantly (1.7 times; p <0.01) The area of the cortex of these lymph nodes increases 1.8 times, compared with the control group (p <0.05).The structural organization of the lymph nodes, which underwent significant transformations during endogenous intoxication, such as preeclampsia, depends on the specificity of the drained areas of organs and tissues [12,13,14,15]. The functional connection between the lymph nodes dictates the need to study the features of their structural organization, depending on the belonging to the topographic and anatomical group.The morphometric characteristics of the functional morphology of LN are substantially supplemented by quantitative indices and coefficients used by different authors [12,14]. In particular, the cortical-cerebral (C/C) index has a certain value, based on the values of which three types of LN are distinguished - fragmented, compact and intermediate, which is of serious importance in assessing the drainage function of the LN. In addition to the C/C index, such derived quantitative indicators as the T / B ratio and the parenchymal-stromal index of the cortex and medulla of the LN were also calculated. The so-called follicular coefficient [15], which is the ratio of the number of secondary LFs to the number of primary ones, reflects the severity of the immune response of the humoral type, carried out due to the development of HC, where the processes of plasmatization of B-lymphocytes and antitelopoiesis take place.At present, digital photography and various computer programs have become quite widespread, which make it possible to automate the process of morphometric study of LN and significantly reduce the complexity and duration of research? Using the considered morphometric methods, the state of various tissue structures of the LN was studied. Their list is different for different authors, depending on the objectives of the study. In many works, the morphometric characteristics of all components of the parenchyma and stroma of the LN are given. Other researchers limited themselves to a few parameters in a variety of combinations. Critically evaluating the presented literary material concerning the morphometric study of LN, we should, in our opinion, recognize the most attractive algorithm used in the work of the employees of the Research Institute of Clinical and Experimental Lymphology of the Siberian Branch of the Russian Academy of Medical Sciences. In this case, the point counting method determines the specific areas (as a percentage of the total cut area of the LN) of such structures as the capsule, subcapsular sinus, CP, primary LF (F1), secondary LF (F2), PC, pulp cords, cerebral sinus. If necessary, the C/C index and the follicular coefficient (F2 / F1 index) are calculated. The indicated algorithm allows one to fairly objectively judge the level of both main functions of the LN - immune and drainage. However, all of the listed methods of morphometric study of LN, each of which has its own pros and cons, still insufficiently clearly reflect the subtler nuances of changes in the morphological substrate of the immune function of LN in different conditions of the organism's existence. We are talking about the severity and ratio of immune responses of the humoral and cellular types, as well as about the processes due to which the level of functional immune activity of the humoral type of LU changes - hyper- / hypoplasia of Lf in general or hyper- / hypotrophy of individual Lf. To avoid the noted drawbacks, in our opinion, the proposed new approach to the problem, based on the experience of other researchers, in particular, given in the review part of the article, and on our own developments, allows us. It should be noted here that the proposed method is intended to study the morphological basis of only the immune function of the LN at the tissue level, leaving aside the drainage function of the organ. Therefore, the number of structural objects of the LN tissue subjected to morphometry is significantly reduced. This also has a certain plus, since it saves research time and reduces its labor intensity.
6. Conclusions
- - In the sub-splenic lymph nodes, a significant expansion of the area of the paracortex was noted and was almost twice as large as in the control of the same group. In these lymph nodes, a significant decrease in the area of the cortex and sinuses of the medullary layer was also noted.- In the paratracheal lymph nodes, the area of the lymphoid follicles, paracortex and pulp cords expanded, while the occupied area of other zones decreased.- Morphometric indicators of various morphofunctional zones of lymph nodes of different localization of deceased women in the control group differ from each other.- Cortical-cerebral index and follicular index are the main morphometric indicators showing the true functional state of the lymph node.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML