Allaberganov Dilshod1, Boboev Khamza1, Khojanazarova Saulexan2, Akbar Khaitov3, Tajimova Nargiza2
1Department of Pathological Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan
2Department of Anatomy and Clinical Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan
3Department of General Surgery and Orthopedics Traumatology, Urgench Branch of Tashkent Medical Academy, Khorezm, Uzbekistan
Correspondence to: Allaberganov Dilshod, Department of Pathological Anatomy, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this study, newborn with sepsis from 8–28 days in the late neonatal period developed morphological changes specific to both primary and secondary immune responses, depending on the gestational period. Of these, the percentage of follicular hyperplasia was determined, followed by paracortical hyperplasia, sinus histiocytosis, stromal cell proliferation, mixed-type hyperplasia, and lymph node fattening.
Keywords:
Late neonatal period, Infants, Sepsis, Lymph nodes, Morphology
Cite this paper: Allaberganov Dilshod, Boboev Khamza, Khojanazarova Saulexan, Akbar Khaitov, Tajimova Nargiza, Morphological Changes of Newborn Lymph Nodes in Late Neonatal Sepsis Period, American Journal of Medicine and Medical Sciences, Vol. 11 No. 7, 2021, pp. 547-551. doi: 10.5923/j.ajmms.20211107.10.
1. Introduction
The relevance of the research currently underway in the study of neonetal sepsis is that the lymph nodes identify the pathogens that enter the body as the main peripheral organs of the immune system [1,2,3,4]. Distinguishes pathogens, senses the organism's infection, adapts to it, detects its antigenicity and fights it. According to the World Health Organization (WHO), “Global epidemiological indicators of neonatal sepsis are not accurate, but 1.2 million out of 3 million newborns are infected with sepsis every year” [1]. As a result, “it stimulates the body's nonspecific response, then the innate immune response, and finally the highly specialized acquired immune response” [2].Late neonatal sepsis is the most common of the pathomorphological changes in the lymph nodes, follicular hyperplasia manifests as a morphological manifestation of a secondary immune response depending on the postnatal period in children, the mechanism of infection, and the morphofunctional status of the lymph nodes [5,6,7,8]. In this case, the process of lymphofollicular hyperplasia was observed to be strong or weak, depending on the level of morphofunctional development of the lymph node. If the lymph nodes were relatively immature, it was observed that they had severe hyperplasia, although the lymphoid follicles in the cortical layer were rare [9]. In this case, it was found that the primary follicles in the lymph nodes become hyperplasia and become secondary follicles. Lymphoid follicles are of different sizes and different morphofunctional conditions, and their hyperplasia is primarily due to the proliferation of the germinative center, reticular cells, macrophages and lymphocytes in it, relatively starving [10,11]. They were found to contain macrophages and centrablasts with increased phagocytic activity [12,13].
2. Purpose of the Research
Evaluation of pathomorphological changes that develop in the main morphofunctional areas of lymph nodes during late neonatal sepsis and the development of an algorithm for clinical and morphological diagnosis.
3. Methods
Morphological, morphometric immunohistochemical and statistical research methods were used in the study.
4. Discussion and Results
Morphological study of lymph nodes in infants who died of sepsis in the late neonatal period shows that, in contrast to early neonatal sepsis, the following pathomorphological changes were detected: lymph node hypoplasia - 2.7%, follicular hyperplasia - 41.5%, paracortical hyperplasia - 24.6%. sinus histiocytosis - 18.7 percent, stromal cell proliferation -6.2 percent, and mixed-type hyperplasia - 4.5 percent, lymph node fattening 4.2 percent.All morphofunctional areas of lymphoid follicles, the germinative area, the V-lymphocyte ring, the mantle, and the interstitial areas of the follicles, are also found to be hyperplasia. Lymphofollicular hyperplasia was strongly present when the lymph nodes were well developed. Germinative areas were found to consist of light-colored, activated cells of round or oval shape (Fig. 1).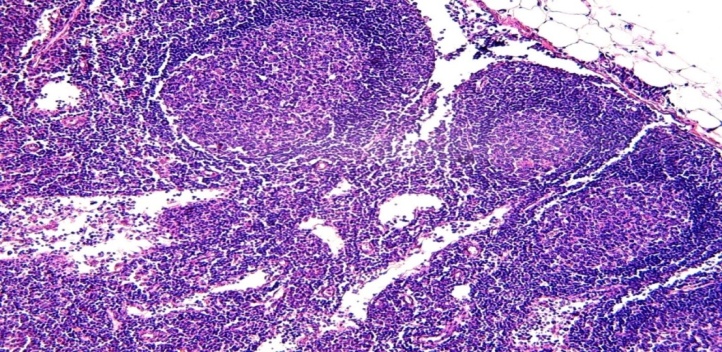 | Figure 1. A 15-day-old infant who died of sepsis in the late neonatal period had a lymph node. Severe hyperplasia of the lymphoid follicles of the cortex, the appearance of germinative centers. Paint: G-E. X: 10x10 |
The lymphocytic ring has a thicker side of the lymphoid follicle facing the peripheral outer membrane, a thinner side between the follicles and the side facing the paracortical area. It is found that the proliferation of lymphocytes also spreads to the extrafollicular areas, causing lymphocytic infiltration of both the peritoneum and the paracortical area. It is observed that a large number of proliferated lymphocytes, lymphoblasts, immunoblasts also enter the sinus cavity, where they bind to macrophages.In some cases, lymphoid follicles also appeared in the paracortical area of the lymph node and in the stratum corneum, leading to severe hyperplasia (Fig. 2).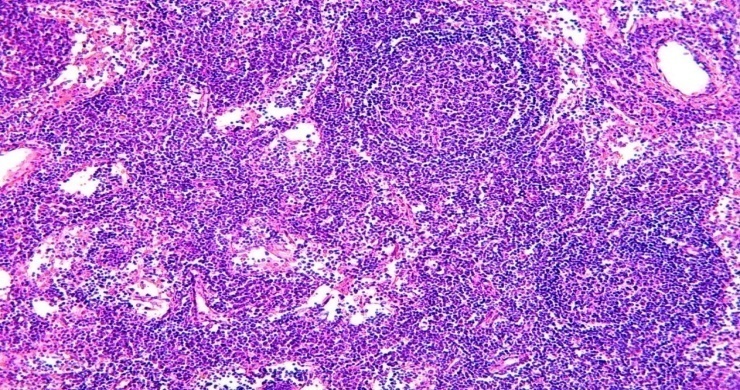 | Figure 2. 15-days-infant who died of sepsis in the late neonatal period had a lymph node. Occurrence of lymphoid follicles in the lymph node core layer. Paint: G-E. X: 10x10 |
In this case, the resulting lymphoid follicles are of different sizes, their mantle area is connected to the nuclei of the interstitial space, and strongly proliferated lymphocytes are also infiltrated into the interstitial barriers of the sinuses. The germinative center in the lymphoid follicles in these areas is also of different sizes, probably due to the lack of reticular cells in it, macrophages. Therefore, the composition of the germinative centers, which appeared late, was mainly composed of lymphoblasts and centrablasts.In the process of lymphofollicular hyperplasia, the transformation of primary follicles into secondary follicles was found to differ from hyperplasia secondary lymphoid follicles in their structure and morphofunctional status. The composition of the germinative center formed in hyperplasia primary follicles differs from the center of secondary lymphoid follicles. It is observed that mainly lymphoblasts, centrablasts and immunoblasts are formed. Reticular cells and macrophages are rare. Another difference is that in the V-lymphocytic ring of primary lymphoid follicles, the lymphocytes are dense and arranged in rows. In cases of late neonatal sepsis complicated by septic shock and disseminated intravascular coagulation syndrome (DICS), foci of hemorrhage and necrosis have been observed in the lymph nodes, as in all internal organs. Such foci were found to develop mainly in the nucleus accumbens and paracortical area of the lymph nodes (Fig. 3).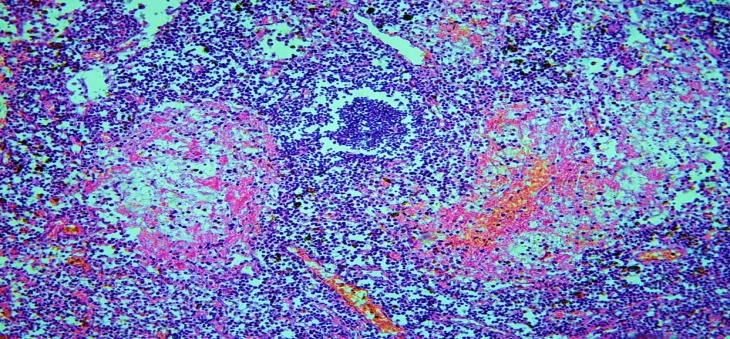 | Figure 3. 17-day-old infant who died of sepsis in the late neonatal period had a lymph node. Occurrence of foci of necrosis in the lymph nodes as a complication of septic shock. Paint: G-E. X: 10x10 |
It was found that the lymph node blood vessels are full, in some of them there are septic thrombi, and as a result of the development of ischemia in the lymph node tissue there are foci of necrosis and hemorrhage. It was observed that the foci of necrosis occupied a large number of areas of different sizes, in which mainly fibrinoid necrosis developed. If the fibrinoid necrosis was located in the center, the presence of macrophages in the peripheral parts of the necrosis and infiltration by lymphocytes was detected (Fig. 4).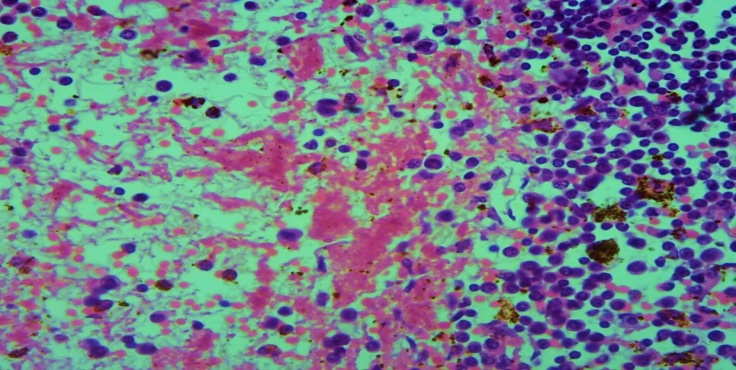 | Figure 4. The top is part of the picture. Fibrinoid necrosis, macrophage reaction around and infiltration of lymphocytes around necrosis. Paint: G-E. X: 10x40 |
It was observed that the lymphoid tissue surrounding the necrosis, especially the lymphoid follicles, was sequestered from the surrounding tissue.Paracortical area hyperplasia is also common among lymph node lesions in late neonatal sepsis. The reason for this is known, the abundance of microorganisms that activate cellular immunity among the pathogens of sepsis. Morphologically, in the lymph nodes, in some cases, the cortical layer is not detected, the paracortical area is defined in the form of a large area (Fig. 5).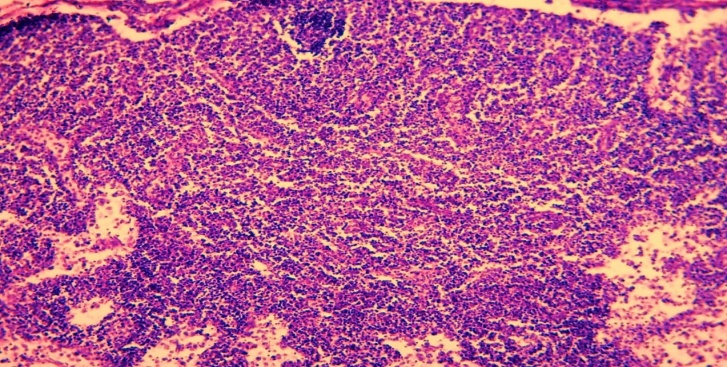 | Figure 5. 9-day-old infant who died of sepsis in the late neonatal period had a lymph node. Paracortical area hyperplasia. Paint: G-E. X: 10x10 |
In other cases, the paracortical area was observed to be hyperplasia and enlargement, growing into the stratum corneum on one side and the cortical layer on the other. As a result, it is determined that the cortical lymphoid follicles are compressed and reduced in size, while the sinuses of the nucleus are narrowed due to compression. The area-enlarged paracortical area was found to be slightly darker on staining, and the soft tufts of interstitial sinuses in the nucleus accumbens appeared to be darker stained.The wall of postcapillary venules located in the paracortical area was hypertrophied and enlarged by endothelial cells, among which migrating lymphocytes were detected, resulting in narrowing of the venous cavity (Fig. 6).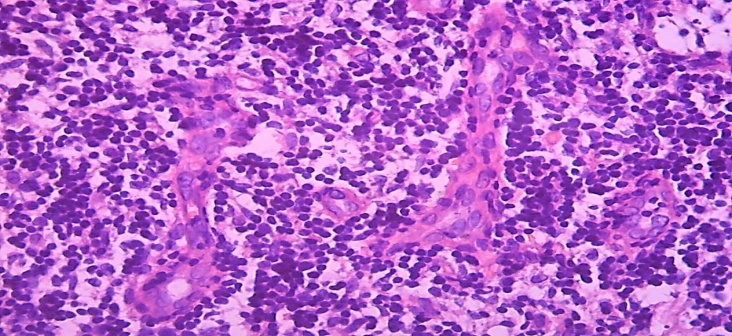 | Figure 6. A 13-day-old infant who died of neonatal sepsis had a lymph node. Hypertrophy of the endothelium of the wall of the postcapillary venules, migration of lymphocytes, the formation of clusters of lymphocytes around. Paint: G-E. X: 10x40 |
The space between the postcapillary venules is filled with activated small and medium-sized lymphocytes, and they are arranged in separate groups, some of which are grouped around macrophages.In some cases, an increase in the number of postcapillary venules hyperplasia was observed in the paracortical area of the lymph nodes (Fig. 7). It was observed the presence of lymphocytes migrating between the endothelium in the vascular wall, the development of diffuse diapedesis hemorrhage around it. It is found that the nuclei of lymphocytes between the foci of hemorrhage are activated by hyperchromization, the formation of immunoblasts. Another major of the lymph node changes characteristic of late neonatal sepsis is mixed-type hyperplasia. In this case, it was observed that in the lymph node tissue at the same time hyperplasia of both cortical lymphoid follicles and the paracortical area, and sometimes soft tufts between the sinuses of the nucleus accumbens. The cause of this type of pathomorphological changes should definitely be sought from the pathogens of sepsis.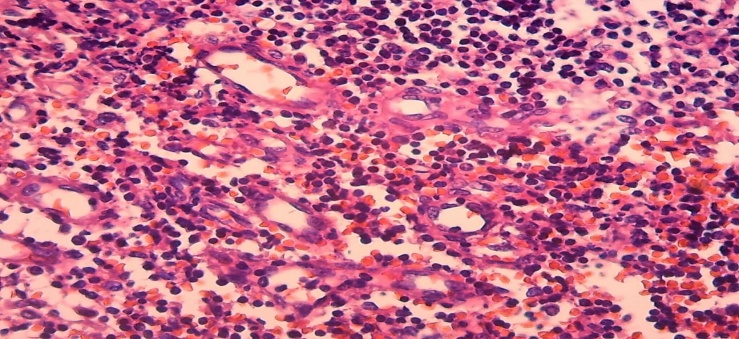 | Figure 7. 11-day-old infant who died of sepsis in the late neonatal period had a lymph node. Postcapillary venous hyperplasia, diapedesis hemorrhage. Paint: G-E. X: 10x40 |
As mentioned above, the reasons for the development of neonatal sepsis in young children are often confirmed to be a mixed infection. It is natural that the antigens that appear in the body of children in mixed infection are also different. Therefore, both cellular and humoral immunity develop at the same time. Accordingly, in the organs of the immune system, including the lymph nodes, both immune-specific morphofunctional areas undergo hyperplasia. Morphologically, simultaneous hyperplasia of the cortical lymphoid follicles in the lymph nodes, the appearance of germinative areas in them was observed. Similarly, the paracortical area was also found to be hyperplasia and dilated, and in some cases even lymphoid follicles (Fig. 8).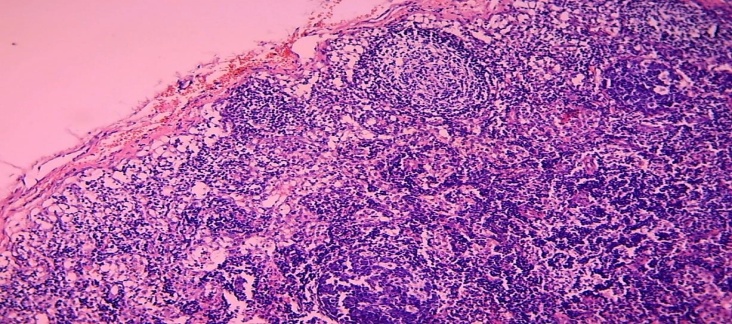 | Figure 8. 11-day-old infant who died of sepsis in the late neonatal period had a lymph node. Mixed type, hyperplasia of both cortical lymphoid follicles and paracortical area. Paint: G-E. X: 10x10 |
At the same time, hypertrophy and hyperplasia of the reticular stroma structures in the paracortical area were observed simultaneously. Among them, it was observed that lymphocytes activated by hyperchromization of the nucleus formed clusters of different sizes.In some cases of this type of mixed type hyperplasia, a sharp enlargement of the paracortical area, in which the postcapillary venules are both hyperplasia and wall cells hypertrophied (Fig. 9). It was observed that the postcapillary venules grew towards both the cortex and the nucleus accumbens. In some cases, postcapillary venules have also been found to grow into soft tufts of interstitial sinuses in the nucleus accumbens. When viewed under a large microscope lens, a strong proliferation of postcapillary venules and the endothelial cells that make up their wall was observed in the paracortical area. As a result, a large number of endothelial cell buds and newly formed postcapillary venules were identified in this area (Fig. 10).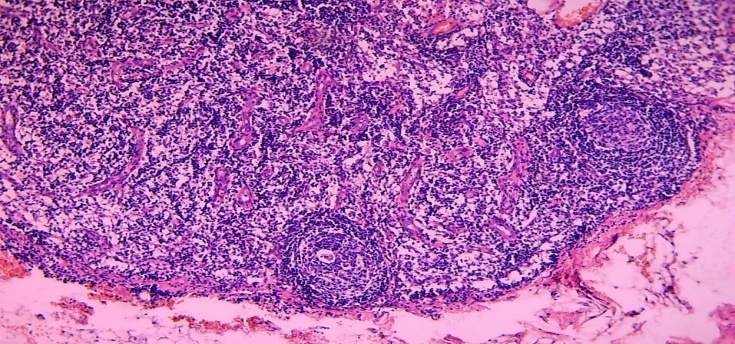 | Figure 9. 15-day-old infant who died of sepsis in the late neonatal period had a lymph node. Expansion of the paracortical area, in which the proliferation of postcapillary venules. Paint: G-E. X: 10x10 |
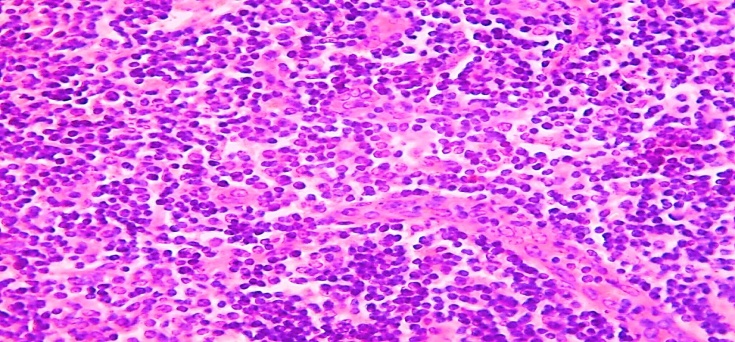 | Figure 10. 15-day-old infant who died of sepsis in the late neonatal period had a lymph node. Proliferation of the endothelium of postcapillary venules, the emergence of new vascular nuclei. Paint: G-E. X: 10x40 |
In mixed-type hyperplasia, the lymphoid follicles in the cortical layer of the lymph node undergo specific hyperplasia. This was due to the enlargement of the germinative center in the center of the lymphoid follicle, the proliferation of reticular cells, macrophages and lymphoblasts in it (Fig. 11). It was found to contain destroyed cells, especially in the peripheral parts of the cells vacuolated, the nuclei underwent karyorexis and karyopycnosis. It was observed that a peculiar ring of small lymphocytes formed around the germinative center, and the lymphocytes in it were arranged in rows.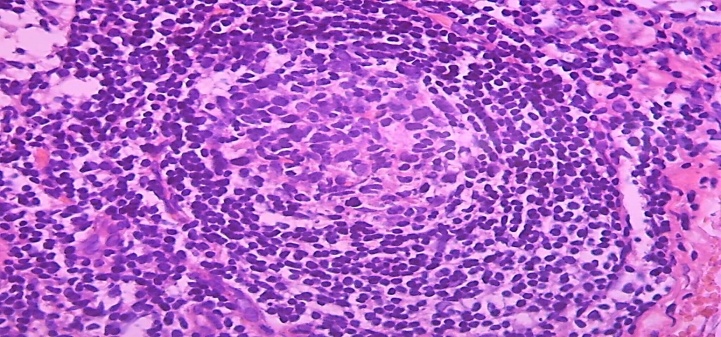 | Figure 11. 15-day-old infant who died of sepsis in the late neonatal period had a lymph node. Proliferation of the cortical lymphoid follicle in mixed-type hyperplasia. Paint: G-E. X: 10x40 |
Another peculiar aspect of the morphological study of the lymph nodes of infants who died of sepsis in the late neonatal period revealed that it was manifested by the development of strong histiocytosis in the sinuses of the lymph node core layer. At the same time, it was observed that the sinuses of both the subcapsular and the stratum corneum of the lymph node are strongly dilated, filled with various cells. In these sinus cavities, macrophages of various morphofunctional levels, destroyed cell and tissue fragments, granular and mononuclear leukocytes were detected in most cases. Hence, the filling of the sinus cavity from the proliferation of hematogenous and histiogenic cells involved in the inflammatory process is called sinus histiocytosis. Similar to the pathomorphological changes in these sinuses, the interstitial sinuses are also found to be enlarged by soft tufts and infiltrated densely with lymphoid and histiocytic cells.In some cases, sinus histiocytosis was followed by severe swelling, which resulted in destruction of the sinus cavities, rupture, and spread to the wall of the connective tissue trabeculae and vessels surrounding the tumor and its cells.It was found that the soft tufts between the sinuses were compressed due to the tumor, and the lymphoid cells in them condensed to form a follicular structure. A study of the state of sinus histiocytosis in a large objective microscope showed that the sinus cavity was filled with both activated and destroyed hematogenous and histiogenic cells at different levels. Most of them were macrophages.Macrophages were found to vary in size, morphofunctional status, and phagocytosis. In the cytoplasm of most of them, phagocytosed cell fragments, apoptotic lymphoid cells were detected. In addition, lymphoid cells of various morphofunctional levels were detected in the sinus cavity, most of which consisted of small lymphocytes. The endothelial cells that make up the sinus wall have also undergone varying degrees of pathomorphology. Most were swollen, desquamated, and displaced, resulting in fusion with hemato-histiogenic cells in the cavity.
5. Conclusions
The following conclusion can be drawn if we analyze the pathomorphological data in the lymph nodes of children who died of sepsis in the late neonatal period in general and in comparison with each other. Changes in the lymph nodes were found to occur primarily due to premature or immature birth of infants, the degree of maturation of the lymph nodes, and the degree to which the sepsis process developed. A small number of children who died of late neonatal sepsis had a primary, congenital, hypoplasia condition in the lymph node. The most common of all detected pathomorphological changes was follicular hyperplasia of these lymph nodes, with an average incidence of 41.5%. Next, paracortical hyperplasia of the lymph nodes - 24.6%, sinus histiocytosis - 18.7%, stromal cell proliferation - 6.2%, and mixed-type hyperplasia - 4.5%, lymph node fattening - 4.2%.
References
| [1] | Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA2016; 315: 801-10. |
| [2] | Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. The Lancet Respiratory medicine 2018; 6(3): 223-30. |
| [3] | Ашиткова Н.В. Diagnostic and prognostic value of lymphopenia in neonatal infections Abstract of Ph.D. thesis 2009, Moscow. |
| [4] | Matics TJ, et al. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children JAMA Pediatrics — | November 02, 2017. |
| [5] | Mohamed El-Mashad G. Et all. La escala pediatrica de evaluacion del fallo multiorganico secuencial (psofa): una nueva escala de prediccion de la mortalidad en la unidad de cuidados intensivos pediatrocos. An Pediatr (Barc). 2019 // doi.org/10.1016/j.anpedi.2019.05.018. |
| [6] | Brady MT, Polin RA: Prevention and management of infants with suspected or proven neonatal sepsis. Pediatrics 132: 166-8, 2013. Doi: 10.1542/peds.2013-1310. |
| [7] | Polin RA and the Committee on Fetus and Newborn: Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics 129: 1006-1015, 2012. Doi: 10.1542/peds.2012-0541. |
| [8] | Gijs T.J. Van Well,  1,4 Leonie A. Daalderop, 1 Tim Wolfs, 2,5 and Boris W. Kramer 3,5 Human perinatal immunity in physiological conditions and during infection Mol Cell Pediatr. 2017 Dec; 4: 4. Published online 2017 Apr 21. Doi: 10.1186/s40348-017-0070-1. 1,4 Leonie A. Daalderop, 1 Tim Wolfs, 2,5 and Boris W. Kramer 3,5 Human perinatal immunity in physiological conditions and during infection Mol Cell Pediatr. 2017 Dec; 4: 4. Published online 2017 Apr 21. Doi: 10.1186/s40348-017-0070-1. |
| [9] | Wynn J. L. Defining neonatal sepsis // Curr. Opin. Pediatr. 2016; 28 (2): 135–140. |
| [10] | Zaidi A. K., Ganatra H. A., Syed S. At al. Effect of cause managements of neonatal mortality due to sepsis and pneumonia // BMC Public Health. 2011; 11 (Supl 3). |
| [11] | Lawn J. E., Blencowe H., Oza S. Et al. Every newborn: progress, priorities, and potential beyond survival // Lancet. 2014; 384 (9938): 189–205. |
| [12] | Gurevich P1, Ben-Hur H, Czernobilsky B, Nyska A, Zuckerman A, Zusman I. Pathology of lymphoid organs in low birth weight infants subjected to antigen-related diseases: a morphological and morphometric study. Pathology. 1995 Apr; 27(2): 121-6. |
| [13] | Gurevich, P., Nyska, A., Czernobilsky, B., Lifschtz-Mercer, B., Zuckerman, A., and Zusman, I., 1997, Nucleolar organizer regions in the splenic lymphoid cells in low-birth-weight and full-term fetuses and newborns with pneumonia and sepsis, J. Histotechnol., 20, 127. |









 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



 1,4 Leonie A. Daalderop, 1 Tim Wolfs, 2,5 and Boris W. Kramer 3,5 Human perinatal immunity in physiological conditions and during infection Mol Cell Pediatr. 2017 Dec; 4: 4. Published online 2017 Apr 21. Doi: 10.1186/s40348-017-0070-1.
1,4 Leonie A. Daalderop, 1 Tim Wolfs, 2,5 and Boris W. Kramer 3,5 Human perinatal immunity in physiological conditions and during infection Mol Cell Pediatr. 2017 Dec; 4: 4. Published online 2017 Apr 21. Doi: 10.1186/s40348-017-0070-1.