-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(6): 482-489
doi:10.5923/j.ajmms.20211106.05
Received: May 12, 2021; Accepted: Jun. 4, 2021; Published: Jun. 15, 2021

Changes in Bone Metabolism in Juvenile Hyperthyroidism
Muratova Shakhlo
Republican Specialized Scientific-Practical Medical Center of Endocrinology Named after Academician Ya.Kh. Turakulov, Tashkent, Uzbekistan
Correspondence to: Muratova Shakhlo, Republican Specialized Scientific-Practical Medical Center of Endocrinology Named after Academician Ya.Kh. Turakulov, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The development of the skeletal system occur during childhood. Thyroid hormones play an important role in the skeleton's normal growth, maturation, and maintenance of the structure and mass of bones. Aimed: identify abnormalities in bone mineral density and the level of calciotropic hormones in juvenile hyperthyroidism to further improve the diagnosis of hyperthyroidism complications on bone metabolism. Methods: The study was conducted by 23 health controls and 71 children and adolescents with hyperthyroidism. All studies were conducted in the Republican Specialized Scientific-Practical Medical Center of Endocrinology, Uzbekistan. Thyroid status and thyroid antibodies, osteocalcin, parathyroid hormone, vitamin D, calcium, phosphorus, alkaline phosphatase were determined using a closed-type immunochemistry analyzer Cobas e 411 Hitachi company Hoffman Le Roche (Switzerland) and its reagents. Bone mineral density was evaluated by dual-energy absorptiometry on a Stratos X-ray densitometer from Diagnostic Medical Systems, France. The results: 59.2% of children with hyperthyroidism were found to be underweight, there was a tendency to accelerated linear growth, low weight and body mass index, and 12.7% were tall. In juvenile hyperthyroidism, in comparison with the control, significantly low values of vitamin D and calcium in the blood serum were noted, the mean values of osteocalcin and alkaline phosphatase were substantially higher. Simultaneously, there was no significant difference in the levels of parathyroid hormone and phosphorus in the blood serum in the compared groups. In 45.1% of patients, a decrease in bone mass was diagnosed compared to the age norm. A reliable direct correlation of vitamin D and calcium with bone density was revealed in all X-ray densitometry parameters and a reliable inverse correlation of osteocalcin, alkaline phosphatase and bone mineral density, as well as Z-scores. Osteocalcin had a stronger inverse correlation with all dual-energy X-ray absorptiometry parameters and became a better biomarker than alkaline phosphatase. Conclusions: There is a decrease in bone mineral density in children and adolescents with hyperthyroidism. Changes in the level of calciotropic hormones indicate a deranged bone metabolism. Serum osteocalcin can be used as a biomarker of bone metabolism in children and adolescents with hyperthyroidism. It is recommended to assess the bones' condition during the initial examination of children and adolescents with hyperthyroidism.
Keywords: Bone mineral density, Hyperthyroidism, Dual energy X-ray absorptiometry, Osteocalcin, Parathyroid hormone, Vitamin D, Calcium, Phosphorus, Alkaline phosphatase
Cite this paper: Muratova Shakhlo, Changes in Bone Metabolism in Juvenile Hyperthyroidism, American Journal of Medicine and Medical Sciences, Vol. 11 No. 6, 2021, pp. 482-489. doi: 10.5923/j.ajmms.20211106.05.
Article Outline
1. Background
- The main thyroid hormones in humans are thyroxine (T4) and 3,3',5-triiodo-L-thyronine (T3), the synthesis and secretion of which are regulated by thyroid-stimulating hormone (TSH) [1]. There are two types of thyroid hormone receptors: TRα and TRβ. The genomic mechanism of action of thyroid hormones is mediated by the intracellular binding of T3 to the nuclear receptor, where it activates TRα and TRβ [2]. In the skeleton, TRα is expressed at a higher concentration than TRβ, and it mediates the effect of T3 on bones and cartilage [3]. The nuclear T3 receptor has been found in osteoblast cell lines and osteoclasts, where it directly stimulates bone resorption in vitro [4]. Dysfunction of the TRα gene leads to a delay in bone maturation, while the lack of all TRβ isoforms does not affect bone cells [3]. Also, during the skeleton's development and growth, T3 regulates the rate of differentiation of chondrocytes, suppresses proliferation, and stimulates prehypertrophic and hypertrophic differentiation of chondrocytes [5]. It has been suggested that TSH plays an additional role in bone metabolism, since the TSH receptor, although predominantly expressed in thyroid follicular cells, has been described in other tissues, including osteoblasts and osteoclasts [6]. TSH inhibits the proliferation and synthesis of the matrix, inhibits the differentiation and function of osteoclasts [7], and it is also assumed that TSH is a key negative regulator of bone metabolism, having a direct effect on bone resorption of osteoblasts due to a decrease in the local production of tumor necrosis factor-alpha [8]. However, this does not explain the cause of osteoporosis in patients with hyperthyroidism. Human studies are limited to the effect of varying TSH concentrations on serum markers of bone metabolism.The relationship between thyroid pathology and the state of bone tissue was first noticed as early as 1891 when Recklinghausen described multiple fractures in a patient with untreated thyrotoxicosis [9]. In adults, thyrotoxicosis causes severe osteoporosis and increases fracture risk, but when compensation is quickly achieved, there is no significant decrease in bone mineral density (BMD) [10]. At the same time, in childhood, up to 90% of genetically determined bone mass is accumulated, which determines the strength of the bone throughout a person's life. Although genetic factors account for 60 to 80% of the variability in skeletal bone mass, other health and lifestyle factors can significantly influence bone growth and remodeling [11].Imbalance of bone metabolism in childhood leads to disruption of bone metabolism, changes in the skeleton, profound disorders of bone formation and mineralization [12].The prevalence of Graves' hyperthyroidism in children is approximately 0.02 per cent (1:5000), mainly in the 11 to 15 age group [13,14]. According to the market report of the endocrinological service of the dispensaries of the regions of the Republic of Uzbekistan for 2016, 58 children and 90 adolescents were registered with thyrotoxicosis, which is 0.6 and 5.0 cases, respectively, per 100,000 population of this age [15]. Published data on the frequency of distribution of reduced mineral density in children in the Republic of Uzbekistan, we are not ours.However, there is very little information regarding the effect of juvenile hyperthyroidism on bone metabolism; the mechanisms of the pathogenesis of osteoporosis in children with thyroid dysfunction are still not fully understood. Various aspects of the action of thyroid hormones on bone tissue continue to be studied to this day. Although the link between hyperthyroidism and impaired bone metabolism in adults is undeniable, there is little research on the effects of thyroid hormones on bone development in children and adolescents with hyperthyroidism [16,17].Simultaneously, questions about the prevalence of reduced BMD remain poorly understood; data on the content of markers of bone metabolism in patients with thyrotoxicosis in childhood and adolescence are contradictory. Simultaneously, late detection, incomplete diagnosis, and delayed therapy of bone metabolism disorders can negatively impact the formation of the body of children as a whole.This study aimed to identify abnormalities in bone mineral density and the level of calciotropic hormones in juvenile hyperthyroidism to further improve the diagnosis of hyperthyroidism complications on bone metabolism.
2. Material and Methods
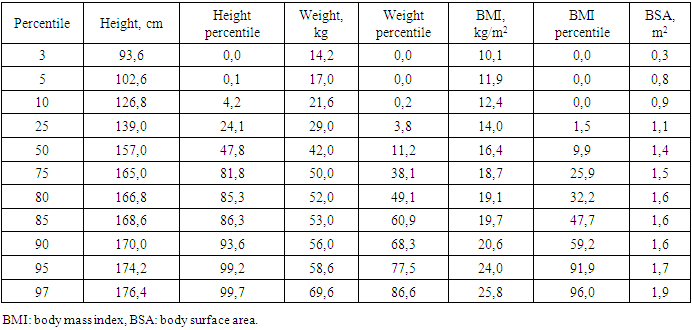
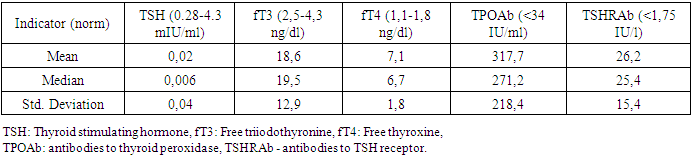
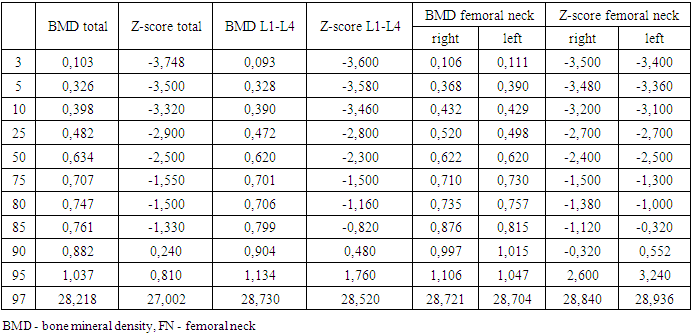
- The study included 71 children and adolescents with the manifest, laboratory-confirmed endogenous hyperthyroidism who sought medical help from a pediatric endocrinologist at the clinic of Republican Specialized Scientific-Practical Medical Center of Endocrinology named after academician Ya.Kh. Turakulov (RSSPMCE) for 2018-2020. Inclusion criteria were childhood, uncompensated hyperthyroidism (TSH < 0.28 mIU/l, wT4 > 1.8ng/dl and wT3 > 4.3ng/dl) of various endogenous etiology (Graves' disease, nodular/multinodular toxic goiter, thyroid cancer with hyperthyroidism). Of these, 85.9% (61) were with newly diagnosed hyperthyroidism, 14.1% (10) - with recurrent hyperthyroidism. Among children and adolescents with endogenous hyperthyroidism, diffuse goiter was detected in 81.7% (58), nodular/multinodular goiter was diagnosed in 16.9% (12), while 4.2% (3) had thyroid cancer (from 2 of them - papillary cancer, 1 - follicular cancer). Antithyroid therapy with drugs registered in the Republic of Uzbekistan (thiamazole) was recommended to children and adolescents after diagnosing hyperthyroidism in doses depending on age, weight and severity of hyperthyroidism. Subsequently, patients with nodular/multinodular toxic goiter and thyroid cancer, and some adolescents with Graves' disease, underwent surgery and radioiodine therapy. The more detailed treatment of patients with hyperthyroidism will be presented in the next publication.The control group consisted of 23 children and adolescents of the corresponding age, whose parents agreed to the examination, without endocrine pathology, who were not under the influence of risk factors for reduced BMD. Their study was also carried out at the RSSPMCE.The study was carried out following the principles of the Declaration of Helsinki. The study protocol was approved by the Local Ethics Committee of the participating institution. The informed consent of the parents of the children was obtained for the research.Anthropometric measurements.Standing height and weight were measured using a standard medical height meter and weights. The body mass index (BMI) was calculated using the formula weight (kilograms)/height (meter)2. Weight, height, percentiles and standard deviation (SDS) for height, weight and BMI were calculated using the WHO Anthro Plus personal computer software, proposed by the WHO Research Group in 2007, which is used to assess the physical development of children regardless of ethnicity, socio-economic status and type of food [18]. The stage of puberty was evaluated using the Tanner scale [19]. Bodyweight was regarded as excessive with BMI within 85–95 percentile, over 95 - as obesity, up to 3 - underweight.Biochemical assessment.The diagnosis of pathology of the thyroid gland and other systems was established based on anamnesis data, clinical manifestations, biochemical and hormonal laboratory studies, ultrasound of the thyroid gland, fine-needle aspiration biopsy of the thyroid gland according to indications), scintigraphy with Tc99, which were carried out at the Republican Scientific and Practical Medical Center of Endocrinology named after Academician Y. Kh. Turakulov, Tashkent, Republic of Uzbekistan. The level of TSH, free thyroxine (fT4), free triiodothyronine (fT3) was determined in blood serum using a closed-type immunochemical analyzer Cobas e 411 Hitachi from Hoffman Le Roche (Switzerland) for biochemical and immunochemical analysis using its own commercial test kits, antibodies to TSH receptors (TSHRAb), antibodies to thyroid peroxidase (TPOAb), as well as biochemical markers of bone metabolism - osteocalcin (OSC), parathyroid hormone (PTH), vitamin D, calcium, phosphorus, alkaline phosphatase (ALP). Bone density measurement.Osteodensitometry, which is the most preferred method for assessing bone mass and bone mineral density (BMD) in children and adolescents, is considered the "gold standard" for diagnosing bone mineralization disorders. In the children and adolescents, BMD was assessed using dual-energy x-ray absorptiometry (DXA) on a Stratos X-ray densitometer from DMS, France using the standard curves of the Asian race M Pediatrics Rachis, left and right Femur and Total body from DMS normality curves 2003/2004. According to the generally accepted WHO diagnostic criteria for osteoporosis, the measurement results were expressed in absolute BMD values (g/cm2) and the form of a Z-score. Measurements were taken in two standard areas of the skeleton: the lumbar spine L1-L5 and the proximal thigh. When interpreting data for low BMD diagnosis, the Total body index is estimated in terms of standard deviations. In the recommendations of the International Society for Clinical Densitometry, it is indicated that BMD and BMC according to DXA results in children and adolescents can be assessed as low with a Z-score <-2 SD following the age and sex of the child [20,21].Statistical analyses. Statistical analyses were carried out using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY). All results are presented as mean ± SDS unless otherwise stated. Independent t-tests were performed to assess the differences between patients and controls. The coefficient of Pearson’s correlations was used to determine the relationship between BMD Z-scores at each site and the other clinical variables. Statistical significance was defined as p < 0.05 for all tests.
3. Results
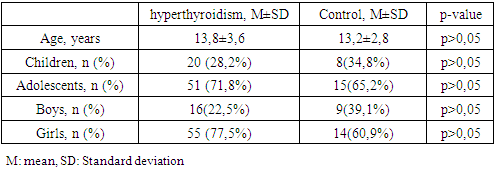
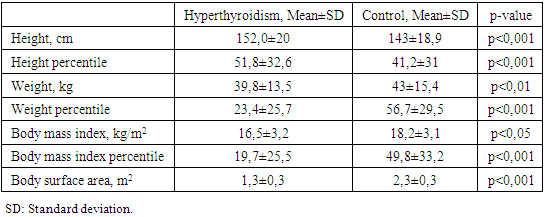
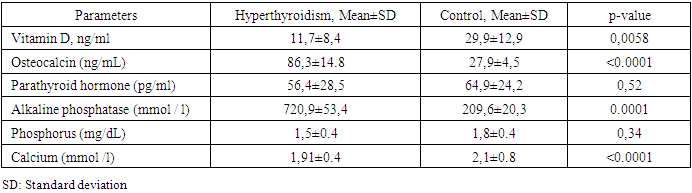
- Demographic, anthropometric and clinical features.The demographic indicators of the studied groups by age, sex and puberty did not significantly differ (Table 1). In the hyperthyroidism group, the average age was 13.8±3., the youngest was 5 years 2 months old, the oldest was 18 years old 1 month old. In the control group, the oldest was 18 years old and 4 months old. The youngest is 4 years 11 months old.
|
|
|
|
|
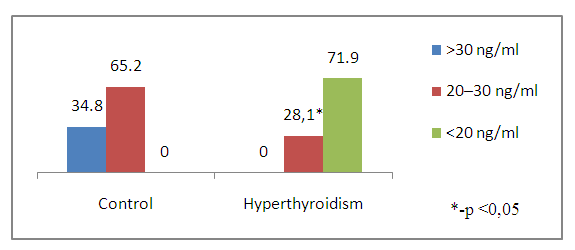 | Figure 1. Vitamin D levels in children and adolescents with hyperthyroidism and control |
|
|
|
4. Discussion
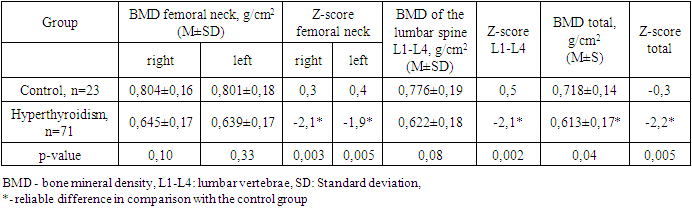
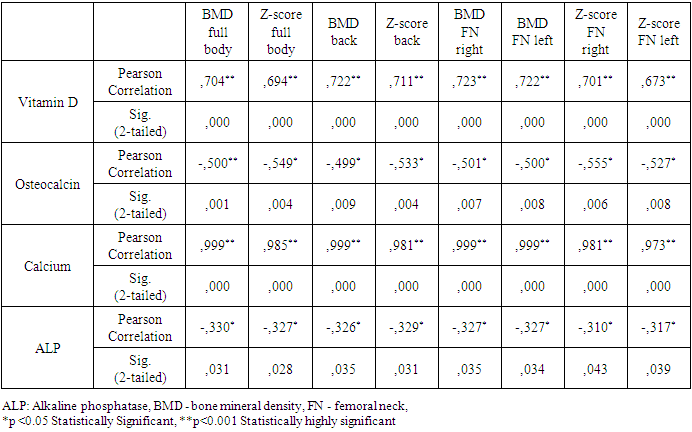
- The period of childhood is of particular importance to the development of the skeletal system, since by the end of puberty the level of bone mass in many parts of the skeleton reaches 86%, and in certain areas - up to 100% of the bone mass of an adult [22]. Bone formation begins in the embryonic period and continues throughout life. The period of active osteohistiogenesis lasts up to 20-25 years [4]. Formation of peak bone mass is a crucial stage in age-related development of the skeleton and a critical physiological moment that determines bone strength throughout the entire subsequent life of a person [23]. Many factors during childhood and adolescence can influence the achievement of maximum peak bone mass. Bone strength, quality and value of bone mineral density are determined mainly by the maximum bone mass, which is achieved in childhood and adolescence. In this case, it is the thyroid hormones in the first two decades of human life that play an important role in the normal growth of the skeleton, maturation and maintenance of the structure and mass of bones, and metabolism [24,25,26]. Therefore, if hyperthyroidism occurs during childhood and adolescence, it can affect bone metabolism throughout life. Juvenile hyperthyroidism is characterized by accelerated skeletal development and rapid linear growth. In severe cases in infants, early closure of the skull sutures can lead to craniosynostosis [27], whereas maternal hyperthyroidism may also be a risk factor for craniosynostosis [28]. These observations additionally demonstrate the pronounced sensitivity of the developing skeleton to the action of thyroid hormones. Hyperthyroidism is an endocrine disorder associated with multisystem effects, but the bone loss in newly diagnosed juvenile hyperthyroidism is rarely reported. Elevated thyroid hormone levels directly accelerate bone remodeling and decrease bone density, which increases the risk of osteoporosis and fractures. These changes can be identified by increased levels of bone markers [29]. In children and adolescents with hyperthyroidism, in comparison with controls, significantly low values of vitamin D and calcium in the blood serum were noted, while the mean values of osteocalcin and alkaline phosphatase were significantly higher. Simultaneously, there was no significant difference in serum PTH and phosphorus levels in the compared groups. Our data do not coincide with the results of the work of Liu H. et al., which indicate that during the period of decompensated hyperthyroidism before treatment, the average levels of phosphorus and PTH in the serum in patients with hyperthyroidism were decreased, the levels of calcium in serum before and after treatment were within normal limits [30].With thyrotoxicosis, the processes of resorption and synthesis of bone tissue are separated, areas of osteoporosis and osteosclerosis are formed; the time of all phases of bone remodeling decreases, and the frequency of occurrence of remodeling sites increases, i.e. the activity of osteoblasts and osteoclasts is increased. The remodeling cycle is reduced by 50%. These changes are disproportionate, resulting in a 10% loss of bone mass in 1 remodeling cycle [7,31]. Densitometry is assessed as the most informative and non-invasive method for the early diagnosis of bone lesions in thyrotoxicosis, which is especially necessary for iodine deficiency regions [32].According to the DXA results, at the time of the study in children and adolescents of both groups, there was no significant difference in BMD of the femoral neck (FN) on the right and left and in the lumbar spine, but BMD of the total body and all Z-score indicators had a reliable difference (Table 6). Simultaneously, in the control group, 1 (4.2%) adolescent (16 years old) was diagnosed with a decrease in bone mass compared with the age norm according to the Z-score. At the same time, in the group with thyrotoxicosis, a low BMD was recorded 10.7 times more often compared with the age norm (32/45.1%) than in the control group, p≤0.001. Similar results have demonstrated Lee H. S. et al. (2020), where 26.8% of children and adolescents with Graves disease had low bone density (LS BMD Z-score<-2.0) [17].Presumably, thyroid hormones increase ALP and OSC expression and stimulate osteoblast proliferation [11,33]. In our research, as shown in Table 8, the most significant direct relationship between BMD and Z-score is noted by vitamin D. But OSC has a stronger inverse correlation with all DXA indicators and is a better biomarker than ALP in assessing the risk of bone loss in patients with juvenile hyperthyroidism. Therefore, elevated serum osteocalcin levels in hyperthyroidism in children and adolescents can be used as a bone metabolism biomarker. Mhaibes S.H. et al. obtained similar values when assessing the correlation between OSC and BMD in adults with hyperthyroidism. In their study the sensitivity, specificity and cut-off point of thyroid profile and bone biomarker for hyperthyroidism patients were estimated by Receiver Operation Characteristic curve (ROC curve) ROC. In the current study, the hyperthyroidism patients had highly significant elevated (p-value<0.0001) OSC and ALP levels as compared to that of control group, results of ROC curve confirmed the validation of OSC as a superior marker in the early detection of bone disease in newly diagnosed hyperthyroidism patients. In addition, serum calcium levels were significantly increased, while no significant difference was observed in the serum phosphorus levels [34].Thus, we have found an increased inverse correlation between an increased level of OSC and ALP in serum with a decreased BMD and Z-score in children and adolescents with hyperthyroidism. Therefore, the assessment of OSC and ALP in serum can help diagnose bone diseases in patients with hyperthyroidism. We hypothesize that OSC is a more reliable biomarker than ALP in assessing the risk of bone loss in newly diagnosed hyperthyroid patients. Children and adolescents with newly diagnosed hyperthyroidism had lower bone mass than their healthy peers. These results suggest that BMD measurement at initial assessment may be necessary for this population. However, given that the study group's sample size is relatively small, and the metabolic status of bone minerals and bone markers after treatment has not been assessed, further research is required. So, we recommend assessing bone health and using serum osteocalcin determination in hyperthyroid children and adolescents as a biomarker of bone metabolism. However, given that the study group's sample size is relatively small, and the metabolic status of bone minerals and bone markers after treatment has not been assessed, further research is required.
5. Conclusions
- In conclusion, our present study highlights the changing of bone mineral density and level of calciotropic hormones in juvenile hyperthyroidism. Osteocalcin levels can be an indicator of the development of osteoporosis in children and adolescents with hyperthyroidism. Long-term follow-up studies to monitor bone mineral status are needed to understand bone metabolism in these patients better.
Declarations
- 1. Ethics approval and consent to participate in the study were approved by the ethical committee of the RCSPMS at the scientific council # 1 dated January 7, 2021.2. Consent for publicationConsent was obtained from parents and guardians to conduct the research and publish data.3. Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.4. Competing interestsThe authors declare that they have no competing interests.5. FundingThe source of funding for the reported study is the RSSPMC at the expense of the applied grant No. PZ-2017091941.6. Authors' contributionsThe development of the study, collection, analysis and interpretation of data, and the writing of the manuscript were carried out by the author, Muratova Sh.T.
ACKNOWLEDGEMENTS
- The author is grateful to Professor Anvar Valievich Alimov for his support in carrying out this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML