Shaislamova M. S.1, Buribaeva B. I.2, Niyazova T. A.3, Zalyalieva M. V.1, Osipova S. O.1
1Republican Specialized Scientific and Practical Medical Center of Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan
2Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
3Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Shaislamova M. S., Republican Specialized Scientific and Practical Medical Center of Epidemiology, Microbiology, Infectious and Parasitic Diseases, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents data on the study Atopic Dermatitis (AD) patients with intestinal parasites. The pprevalence of Enterobius vermicularis, Hymenolepis nana and Giardia lamblia in patients and control individuals was similar, the prevalence of Ascaris lumbricoides was higher (P < 0.05) in AD patients. The infestation with Blastocystis sp. exceeded the infection rate of Chilomastix mesnili, Jodamoeba buetschlii and Endolimax nana by 4.4, 1.8 and 6.6 times, respectively. Total serum IgE level in patients with Blastocystis infection was significantly higher compared to patients without parasites. Anti-parasitic therapy addition to anti-allergic one decreased the total serum IgE level in all cases and improved patient’s status with the SCORAD index. Intestinal parasites and Blastоcystis sp. can be considered as additional triggers of allergy. Addition of anti-parasitic drugs to the therapy significantly decreased of the total serum IgE level and reducing SCORAD index in AD patients.
Keywords:
Atopic Dermatitis, Intestinal parasites, Blastocystis sp., IgE
Cite this paper: Shaislamova M. S., Buribaeva B. I., Niyazova T. A., Zalyalieva M. V., Osipova S. O., Prevalence and Possible Association of Intestinal Parasites in Patients with Atopic Dermatitis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 6, 2021, pp. 476-481. doi: 10.5923/j.ajmms.20211106.04.
1. Introduction
Atopic Dermatitis (AD) is a systemic multifactorial disease involving genetic, immunological and environmental factors. Clinically, AD can progress from a skin disease to food allergy, allergic rhinitis and bronchial asthma, a phenomenon called the “atopic march” [1]. Despite the established mechanisms: damage and dysfunction of the epithelial skin barrier, mediating the penetration of allergens and proteins of microorganisms and IgE-sensitization, imbalance of Th1 / Th2 response, lack of antimicrobial peptides, causing an increased risk of bacterial (mainly Staphylococcus aureus) and viral (dominated by Herpes zoster) infections, the pathogenesis of AD remains largely unclear [2,3]. The approaches to studying the pathophysiology of allergic diseases (AD, food allergy, asthma, allergic rhinitis) are expanding. The factors that initiate and/or pre-dispose to the development of AD are considered: in addition to the above: pathological changes in the intestine, organ-specific structure of the microbiome and deviations in its composition [4]. Changes in the bacterial part of intestinal microbiota are intensively studied, and they are usually considered as the first stage in the development of AD and atopic march. Patients with AD are characterized by low levels of Bifidobacterium and Bacteroides and increased Enterobacteriaceа, colonization of Clostridium difficle is observed.The data on the relationship between the intestinal microbiota and the state of the intestinal barrier with the development of allergic diseases are growing [3]. Microbiota changes can affect the severity of the disease, its duration and response to treatment [5,6].Bacterial part of the microbiota has been mainly studied There are essentially no studies on the role of intestinal protist fauna in the induction of allergic reactions, and the hygienic theory of Strachan D. (1989) [7] on the suppression of allergic and inflammatory processes by helminths remains largely controversial. Santiago et al. (2012) [8] showed an increase in the incidence of bronchial asthma, allergic rhinitis, food allergy, etc. correlates with a decrease in parasitic infections. However, there is evidence contradicting the hygienic theory. The role of Enterobius vermicularis in the development of allergic dermatitis is indicated by Boas et al. (2014) [9]. Infection with Ascaris lumbricoides did not affect the course of AD [10]. The data on the high frequency of helminthiasis in people belonging to the risk groups for inflammatory and allergic diseases, including AD, are presented [11,12]. Intestinal parasitic diseases are widespread and clarification of their role will improve the understanding of AD pathogenesis and, if significant associations are identified, the introduction of mandatory diagnosis of parasitosis with following specific therapy can increase the effectiveness of the treatment.The objective of the present study: to determine the prevalence of intestinal pathogenic parasites and structure of the intestinal protist fauna in AD patients and estimate their role in the etiopathogenesis of the disease.
2. Materials and Methods
Patients with AD applied to the Republican Specialized Scientific Center of Allergology of the Ministry of Health of the Republic of Uzbekistan. A randomized group of 100 AD patients aged 1-12 (59%) and 13-19 years (41%), 58% girls and 42% boys was examined for helminth infestation, Giardia lamblia, and intestinal protist fauna structure. To assess the effect of anti-parasitic therapy on the course of AD, 95 patients with AD with parasitises were additionally selected. Severe, moderate and mild blood pressure was diagnosed in 8%, 67% and 25% of patients, respectively. The control group included 200 persons without complaints from the gastrointestinal tract and the absence of allergologic diseases in the anamnesis, the age and sex structure is similar. The clinical course and its differentiation according to the severity of the disease was carried out in accordance with the SCORAD scale, which combines objective (prevalence of the skin process (A), the intensity of clinical manifestations (B) and subjective criteria (C), including the intensity of itching and sleep disturbance. SCORAD is calculated according to the formula A / 5 + 7B / 2 + C (mild degree - 0-25 points, medium degree - 26-50 points, severe degree - 51-103 points, severe degree) [13].Collection of stool samplesThree stool samples for parasitological examination were taken at 2 days’ interval from AD patients before and after anti-parasitic therapy and control individuals. Stool samples were collected in individual containers, containing 5 ml of Turdiev’s preservative provided conservation and staining of protozoa cysts and eggs of worms for a year. The Turdiev’s preservative includes: 80 ml of 0.2% aqueous solution of sodium nitrite, 10 ml of formaldehyde, 2 ml of glycerin, 8 ml of Lugol’s solution, and 250 ml of distilled water.Stool samples examinationThe parasitological diagnosis was performed by triple coproscopy using formalin-ethyl acetate concentration technique (Truant et al. 1981) and iodine stained smear [14]. The intensity of protozoa was estimated by the number of protozoa in the field of view (ocular x10, objective x40) in iodine stained smears taken before application of formalin-ethyl acetate concentration technique, the number of protozoa was calculated at least in 10 fields of view. 1-2, 3-4 and 5-6 microorganisms in a field of view were considered as infection of low, mean and high intensity respectively. C. parvum were detected using modified Ziehl-Neelsen method. The performers of parasitological diagnosis did not have access to any information about an individual under examination. All information was blinded.Immunological methods: The total serum IgE was determined by the method of enzyme-linked immunosorbent assay ELISA (test system with the corresponding reagent kits JSC Vector-Best (Novosibirsk, Russia). The level of specific IgE to Ascaris lumbricoides was determined using an allergy panel "AlkorBio", St. Petersburg, Russia.AD therapy included 1st and 3rd generation antihistamines, mainly chloropyramine chloride and desloratadine (within 3-4 weeks), topical glucocorticosteroids (for no more than 10 days), emollients Stelatopia, Emolium [2,3,15].Anti-parasitic therapy: with ascariasis, patients received albendazole (single dose of 400 mg), enterobiasis - mebendazole (single dose of 100 mg, repeat after 2 weeks); hymenolepiasis - praziquantel (single dose 25 mg / kg, repeat after 4 days); giardiasis - metronidazole in a daily dose of 15-22.5 mg / kg in three doses for 10 days; blastocystosis - metronidazole in a daily dose of 0.5-2.0 g in 4 doses for 10 days [16]. The criteria for evaluating the effectiveness of anti-parasitic therapy were: elimination of intestinal parasites, a decrease in the total level of serum IgE, and clinical response to complex therapy with the inclusion of anti-parasitic drugs, assessed by the SCORAD scale. The elimination of parasites was controlled twice: immediately after completion and 2 weeks after the end of anti-parasitic therapy. The IgE level and SCORAD scores were determined before treatment, two weeks after anti-allergic treatment and after combination therapy (anti-parasitic and anti-allergic) treatment.Statistical analysis was performed using the Origin 8 software (Origin Lab, Northampton, MA). Results are expressed as mean ± standard error (SEM) for continuous variables and numbers (percent) for categorical data. Data were analysed using odds ratios (OR), 95% confidence interval (CI) means, and Pearson chi-square test for numeric variables. A P value < 0.05 was considered statistically significant.
3. Results and Discussion
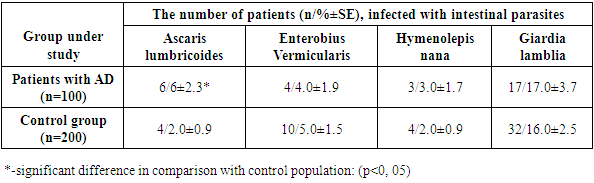
The survey data of the randomized group for pathogenic intestinal parasites are shown in Table 1. The table shows that AD patients are infected with parasites, the most common in Uzbekistan. A significantly higher level of infection in AD patients was determined only for Ascaris lumbricoides, the prevalence of other parasites was at the level of the control group.Table 1. The prevalence of intestinal parasites in AD patients
 |
| |
|
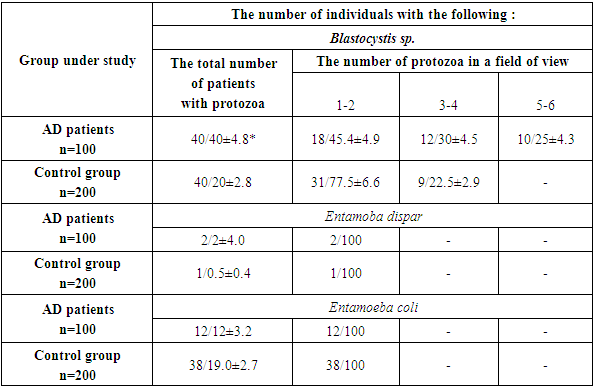
The results of the structure of the intestinal protist fauna determining are given in Tables 2 and 3. The prevalence of Blastocystis sp. in AD patients was twice as high as in the control, respectively 40.9 ± 4.1% and 20.0 ± 2.8% (P < 0.0001). Only in patients with AD in 17.6 ± 5.3% of cases there was a high intensity of infection (≥ 5-6 parasites in the field of view), which was absent in the control. Patients with AD significantly were more often (in 37 ± 6.7% of cases) than in controls (22.5 ± 6.6% of cases) had Blastocystis sp., of medium intensity (3-4 parasites in the field of view) (P < 0.05). Blastocystis sp. low intensity was found significantly less frequently than in the population (P < 0.05).Table 2. Prevalence of C. mesnili, J. buetschlii and E. nana in AD patients (n/%)
 |
| |
|
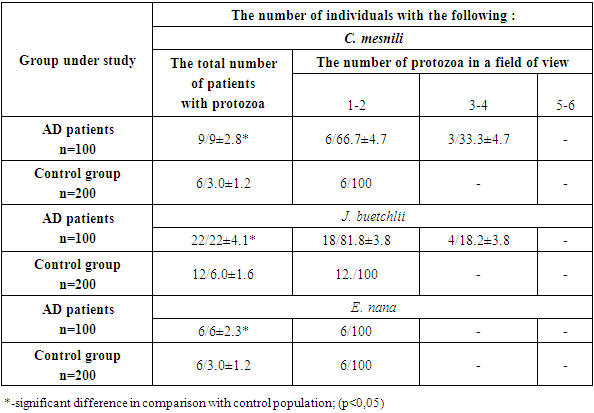
Table 3. Prevalence of Blastocystis sp., E. dispar and E. coli in AD patients (n/%)
 |
| |
|
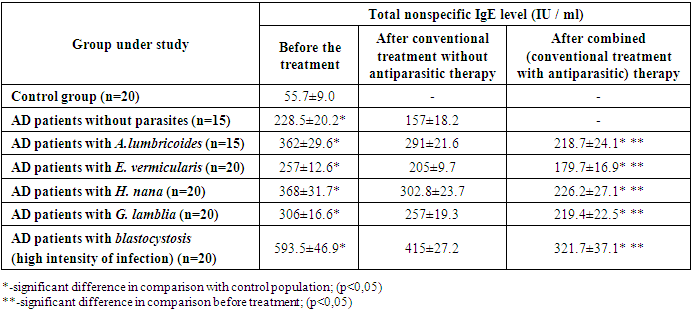
The detection rate of Entamoeba dispar was the same in AD patients and in controls. The opposite tendency was observed for Entamoeba coli - they were detected in patients with AD less often than in controls, respectively 12.0 ± 3.2% and 19.0 ± 2.7% (P < 0.001). Apparently, E. coli is more characteristic of a healthy intestine than of a pathologically altered one.In patients with AD, the infection with Chilomastix mesnili (2.5 times), Jodamoeba buetschlii (3 times) and Endolimax nana (2 times) was found significantly more often than among the population, and in Ch. mesnili and J. buetschlii diagnosed, in addition to low, moderate intensity not found in the population. It should be emphasized that the infestation with Blastocystis sp. exceeded the infection rate of Ch. mesnili, J. buestchlii and E. nana by 4.4, 1.8 and 6.6 times, respectively.The possible role of intestinal parasites in pathogenesis was assessed by the level of total serum IgE in AD patients infected with intestinal pathogenic parasites and Blastocystis sp., Protozoa with debatable pathogenicity, and in AD patients free of parasites and Blastocystis sp. All patients received a combination of 1st and 3rd generation antihistamines, emollients, and, according to indications, topical glucocorticosteroids. The results are shown in the Table 4.Table 4. Total serum IgE level in AD patients infected with intestinal parasites and without them
 |
| |
|
The table shows that the level of IgE in patients with AD without concomitant parasites is significantly higher than in the control group. Concomitant parasites significantly increased IgE levels.Blastocystis sp. (high and medium intensity of infection) induced the highest level of IgE we diagnosed. This result is consistent with the data on the ability of Blastocystis sp. enhance apoptosis of the enterocyte cell line Caco-2, causing permeability of the intestinal barrier, exerting a mucinolytic effect. High intensity of infection and adhesion to enterocytes causes tissue damage [17,18]. Blastocystis sp. identified peroxiredoxins - proteins that play an important role in the survival and virulence of pathogenic eukaryotes and prokaryotes [19]. But since the high intensity of Blastocystis infection was found only in AD patients, and the average intensity (3-4) was more common than in the control, we also determined IgE in AD patients with a low intensity of blastocyst infection. The level of total IgE in 12 blood pressure patients with a low intensity of blastocyst infection was higher than in AD patients free from parasitises, but the differences were insignificant: 254.7 ±34.1 and 228.5 ± 20.2 IU / ml (P > 0.05). Obviously, the level of the IgE response depends on the intensity of the blastocyst infection, which determines the degree of stimulation of the corresponding cells.Additional prescription of antiparasitic therapy led to the elimination of A. lumbricoides, E. vermicularis, H. nana, and G. lamblia in all treated patients. A somewhat different picture was observed in patients with AD with concomitant blastocystosis: the elimination of Blastocystis sp. was observed in 14 patients out of 20, and in 4 the intensity of the infection after the end of the course decreased to 1-2 in the field of vision, in 2 to 3-4.Apparently, this is due to the physiological features of E. vermicularis, a weaker damage to the intestinal barrier compared to other parasites. This assumption is supported by the fact that the lowest level of IgE among the parasites we diagnosed was induced by E. vermicularis. Identification of factors contributing to AD progression can expand the list of mandatory tests and their repetition in patients examination and the range of drugs, based on the known association of AD and intestinal parasitic diseases with a Th2 response and high IgE level [20,21]. We characterized the prevalence of pathogenic intestinal parasites and the structure of the intestinal protist fauna in AD patients during an exacerbation. Prevalence of intestinal parasites in AD patients was at the level of the control group, only Ascaris lumbricoides were detected significantly more often than in the control. Since the patients were examined during the period of exacerbation, it is obvious that parasitic infections don’t inhibit the allergic process, or this effect was weak and do not prevent the exacerbation of the disease. Thus, our data do not fit into the Strahan hygienic theory and consistent with the data of the authors diagnosing intestinal parasites in patients with allergies, including AD [11,12,22]. They also agree with the known data on an increase in the permeability of the intestinal barrier caused by helminths and G. lamblia, which can lead to hypersensitization by allergens of parasites and food molecules in the intestinal lumen due to their translocation across the damaged barrier [21,23,24]. Consequently, parasitic diseases can participate in the pathogenesis of AD, increasing the allergic background and contributing to development of food allergies. We have found that parasites cause a significant increase in the level of total serum nonspecific IgE in AD patients compared to patients free from parasites. The role of parasites as triggers of the allergic process is confirmed by the positive dynamics of the patients state determined using the SCORAD index and by a significant decrease in the IgE level after anti-parasitic drugs inclusion in the complex therapy and parasites elimination.The study data were consistent with the results of Yakovleva et al. 2010 [11] and Qualizza et al. 2018 [22], who diagnosed intestinal parasites, including Ascaris lumbricoides, in AD patients and noted a marked improvement in the condition of patients after the elimination of parasites.The lack of a clear understanding of the etiopathogenesis of AD limits the possibility of influencing trigger mechanisms, therefore, drugs that suppress the atopic and inflammatory process come to the fore: antihistamines, topical use of calcineurin inhibitors and corticosteroids, systemic (corticosteroids, cyclosporine, azathioprine, methotrexate, moisturizers preventing dehydration, reducing itching and Xerosis, reducing the need for topical use of corticosteroids and antibiotics [2,3,25]. Nevertheless, the effectiveness of AD therapy remains rather low. Diagnosis of concomitant parasitoses and their therapy increases the effectiveness of AD treatment, causing a significant decrease in IgE levels and a pronounced clinical response. To a certain extent, our data were consistent with the positive effect of monoclonal antibodies to the IL-4 receptor (dupilumab) on the AD course which reduce the severity of the disease, including itching and improve the quality of life, that is associated with the ability of IL-4 to disrupt the skin barrier [26], and helminths stimulate the Th2 response and the production of IL-4 [16].
4. Conclusions
Intestinal parasites, including Blastocystis sp. infection of high and medium intensity, significantly increase the level of total serum IgE in AD patients. Anti-parasitic therapy improves the condition of patients, significantly reducing the level of IgE and the sum of points according to the SCORAD scale. It is recommended conducting a parasitological examination of AD patients followed by anti-parasitic therapy. It is of special importance in regions endemic on intestinal parasites. Intestinal parasites can be considered as additional triggers of atopy in AD patients.
References
| [1] | Rerknimitr P., Otsuka A., Nakashima C., Kabashima K. 2017, The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 37: 14. |
| [2] | Balabolkin I.I., Bulgakova V.A, Eliseeva T.I. 2017, Atopic dermatitis in children: immunological aspects of pathogenesis and therapy. J Pediatric. №2. 128-135. |
| [3] | Zhu T. H., Zhu T. R., Tran K.A., Sivamani R. K., Shi V.Y. 2018, Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation Review Br J Dermatology. Sep; 179(3): 570-581. |
| [4] | Krempski J.W., Dant Ch.Nadeau K.C. 2020, The origins of allergy from a systems approach // July 20, 2020 DOI: https://doi.org/10.1016/j.anai.2020.07.013. |
| [5] | Kim J.E., Kim H.S. 2019, Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies // J Clin Med. Apr; 8(4): 444. |
| [6] | Han-Na Um, Jin-Ok Baek, Sohyeon Park Eun-Hui Lee et al. 2021, Small intestinal immune-environmental changes induced by oral tolerance inhibit experimental atopic dermatitis. Cell Death Dis. Mar 4; 12(3): 243. |
| [7] | Strachan D. P. 1989, Hay fever, hygiene, and household size. BMJ. Nov 18; 299(6710): 1259-60. |
| [8] | Santiago H.C., et al. 2012, Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin. Immunol. –Jul; 130(1): 248-56.9. |
| [9] | Boas H., Tapia G., Rasmussen T., Ronningen K.S. 2014, Enterobius vermicularis and allergic conditions in Norwegian children. Epidemiology. Infectology. 142, 2114-2120. |
| [10] | Silva M.T., Souza V.M., Bragagnoli G., Pereira T.G., Malagueсo E. 2010, Atopic dermatitis and ascariasis in children aged 2 to 10 years. JPediatr (RioJ). Jan-Feb; 86(1): 53-8. |
| [11] | Yаkovleva V.K. 2010, Influence of the treatment of parasitizes and intestinal dysbiosis on the course of bronchial asthma and other allergic diseases. SPb, 53. |
| [12] | Briggs N., Weatherhead J., Jagannadha K. S., Hotez P.J. 2016, The Hygiene Hypothesis and Its Inconvenient Truths about Helminth Infections. PLoS Negl Trop Dis. Sep; 10(9): e0004944. |
| [13] | Kamasheva G.R., Hakimova R.F., Valiullina S.A. 2010, Methods for assessing the severity of atopic dermatitis in young children. Dermatology. Kazan, Russia, 32-34. |
| [14] | Truant A.L., Elliott S.H., Kelly M.T., Smith J.H. Comparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinc sulfate flotation techniques for detection of intestinal parasites. J Clin Microbiol. 1981 May; 13(5): 882–884 |
| [15] | Hardikova S.A., Dmitruk V.S. 2019, Basic complex therapy of atopic dermatitis: the use of emollients as an important factor in the restoration of the skin barrier Clinical Dermatology and Venereology. 18, № 5. 591-596. |
| [16] | Sergiev V.P., Lobzin YU.V., Kozlov S.S. “Human parasitic diseases” SPb. 639. 2016. |
| [17] | Wu Z., Mirza H., Tan K.S.2014, Intra-subtype variation inenteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7 // PLoS Negl Trop Dis.. 8(5): e2885. |
| [18] | Ajjampur S.S., Tan K.S. 2016, Pathogenic mechanisms in Blastocystis spp. - Interpreting results from in vitro and in vivo studies // Parasitol Int 65(6 Pt B): 772-779. |
| [19] | Angelucci F., Miele A.E., Ardini M, Boumis G, Saccoccia F, Bellelli A. 2016, Typical 2-Cys peroxiredoxins in human parasites: Several physiological roles for a potential chemotherapy target. Mol Biochem Parasitol. (1-2): 2-12. |
| [20] | Haase P., Voehringer D. 2021, Regulation of the humoral type 2 immune response against allergens and helminths. Eur J Immunol. 51(2): 273-279. |
| [21] | Halliez M. C. Buret A.G. 2013, Extra-intestinal and long-term consequences of Giardia duodenalis infections World J Gastroenterol. Dec 21; 19(47): 8974–8985. |
| [22] | Qualizza R. Losappio L.M., Fabiana Furci F. 2018, A case of atopic dermatitis caused by Ascaris lumbricoides infection Clin Mol Allergy – Apr 10; 16: 10. |
| [23] | Garzуn M., Pereira-da-Silva L., Seixas J. et al. 2017, Association of enteric parasitic infections with intestinal inflammation and permeability in asymptomatic infants of São Tomé Island. Pathog Glob Health. – May; 111(3): 116–127. |
| [24] | Zavala G.A., Garcia O.P., M. Camacho M. et al. 2018, Intestinal parasites: Associations with intestinal and systemic inflammation. Parasite Immunol. Apr; 40(4): e12518. |
| [25] | Van Zuuren E.J., Fedorowicz Z., Christensen R., Lavrijsen A.P.M., Arents B.W.M. 2017, Emollients and moisturisers for eczema. Cochrane Database Syst Rev. Feb; 2017(2): CD012119. |
| [26] | Cabanillas B., Brehler A.C., Novak N. 2017, Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol. Aug; 17(4): 309–315. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


