-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(6): 465-470
doi:10.5923/j.ajmms.20211106.02
Received: Apr. 9, 2021; Accepted: May 28, 2021; Published: Jun. 15, 2021

The Clinical Course of Brucellosis, Taking into Account the Phenotype of Acetylation in Patients
Nargiza E. Kadirova, Gulnara A. Ibadova
Department of Children’s Infectious Diseases of the Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
Correspondence to: Nargiza E. Kadirova, Department of Children’s Infectious Diseases of the Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This work aimed to study the relationship of the acetylator phenotype with the features of the clinical course of brucellosis in patients to develop prognostic criteria for the development of complications and indications for the use of antibiotics depending on the degree of N acetyltransferase activity. Materials and methods. The study included 178 patients with various clinical forms of brucellosis. The acetylation phenotype was determined in urine by the method of A.M. Timofeeva modified by G.A. Ponomarev. Results. The results obtained showed that brucellosis in SA has a longer duration with destructive and necrotic lesions of organs such as the myocardium, liver, brain, and joints. Since the process of SA is sluggish, intermediate products of inflammation accumulate in the body, and high endogenous intoxication develops. The transition of an acute process to a chronic one increases. The conducted antibiotic therapy has a positive effect since a high concentration of the antibiotic accumulates in the body, but the toxic effect of the drug on the liver increases. For patients with FA the process proceeds quickly, due to this, endogenous intoxication in patients is low, the antibiotic therapy carried out is temporary, since the antibiotic is released from the body faster and the expected effect is not observed. Due to this, in 24.5% of adults with FA, the acute process becomes chronic. Conclusions: obligatory determination of the type of acetylation phenotype: in cases of detection of a slow type of acetylation, the appointment of combined antibiotic therapy should be controlled depending on the value of ABL to the TA of the joints with cancellation or continuation of treatment and add hepatoprotection to prevent hepatotoxic effect and detoxification therapy; in cases of detection of a rapid type of acetylation, the appointment of a longer course of combined antibiotic therapy, depending on the value of ABL to TA of the joints, cancel or continue treatment.
Keywords: N acetyltransferase, Acetylation phenotype, Patient stratification, Individualization of treatment
Cite this paper: Nargiza E. Kadirova, Gulnara A. Ibadova, The Clinical Course of Brucellosis, Taking into Account the Phenotype of Acetylation in Patients, American Journal of Medicine and Medical Sciences, Vol. 11 No. 6, 2021, pp. 465-470. doi: 10.5923/j.ajmms.20211106.02.
Article Outline
1. Introduction
- Brucellosis is a zoonotic disease whose aetiological agent was first identified by Sir David Bruce in the 1860s [1,2]. Despite being long recognized, the disease continues to be prevalent and afflicts humans and domestic, and other animals in many countries around the world. Most affected are the Eastern Mediterranean basin, the Middle East, the Arabian peninsula, Mexico, Central, and South America, Central Asia, and the Indian subcontinent [3,4].The disease results in a wide range of significant veterinary and public health problems, and economic loss. Increased business and leisure travel to endemic countries has led to diagnostic challenges in areas where brucellosis is uncommon, especially when the presentation is unusual [5–8].Because of the low infectious transmittable dose (≤102 organisms), the protracted pathogenesis and disease, and the possibility of it being used in biological weapons, Brucella was classified as a category B ‘select agent’ [9,10]. Brucellosis is a zoonotic disease whose aetiological agent was first identified by Sir David Bruce in the 1860s [1,2]. Despite being long recognized, the disease continues to be prevalent and afflicts humans and domestic, and other animals in many countries around the world. Most affected are the Eastern Mediterranean basin, the Middle East, the Arabian peninsula, Mexico, Central, and South America, Central Asia, and the Indian subcontinent [3,4].The disease results in a wide range of significant veterinary and public health problems, and economic loss. Increased business and leisure travel to endemic countries has led to diagnostic challenges in areas where brucellosis is uncommon, especially when the presentation is unusual [5–8].Because of the low infectious transmittable dose (≤102 organisms), the protracted pathogenesis and disease, and the possibility of it being used in biological weapons, Brucella was classified as a category B ‘select agent’ [9,10].The persistent worldwide prevalence of human brucellosis causes serious public health concerns and economic loss to communities. The multisystem involvement and the protean and unusual clinical presentations of the disease pose significant diagnostic challenges. The clinical features are non-specific and can overlap with a wide spectrum of other infectious and non-infectious diseases, leading to brucellosis being labeled the ‘disease of mistakes’. Protracted chronicity and serious complications can result and mislead physicians onto a path of costly laboratory and radiological investigations. To reach a diagnosis clinicians must use a wide range of non-specific routine hematological and biochemical tests in addition to Brucella-specific assays.The predominance of chronic forms in the structure of the morbidity of brucellosis, the recurrent course of the disease, and the incomplete effectiveness of traditional drugs determine the urgency of the problem of brucellosis therapy [13,14].To improve the quality of diagnosis, treatment, and prognosis of the course of acute brucellosis, it is necessary to identify genetically determined facts that affect its outcome and determine the individual approach to the treatment of each patient. With an individual approach to the treatment of a patient, the doctor must consider the metabolism of pharmaceuticals. According to the literature, it was found that the degree of activity of N acetyltransferase is genetically determined for each person, and the enzyme itself is involved in the metabolism of xenobiotics [11,12,15].Following the aim of the study, the following tasks were set: to study the features of the statistical regularities of the distribution of patients by the degree of N acetyltransferase activity in various clinical forms of brucellosis; to establish whether there is a relationship between the characteristics of the clinical course, the severity of the disease and the rate of N acetylation in patients with brucellosis; to assess the role of the acetylation phenotype as a marker of predisposition to the development of complications of acute brucellosis. The studies were carried out at the RIEMID (Research Institute of Epidemiology, Microbiology and Infectious Diseases) clinic of the Ministry of Health of the Republic of Uzbekistan for the period from 2015 to 2018. Patient groups were selected by random sampling as they were admitted to the hospital.The diagnosis of brucellosis was verified based on an epidemiological history, anamnestic and clinical studies, laboratory parameters, and identification of the pathogen by bacteriological examination per Order No. 37 of the Ministry of Health of the Republic of Uzbekistan dated January 23, 2015 "On measures to reduce the morbidity of especially dangerous infections in the Republic of Uzbekistan".We set the task of studying the effectiveness of the therapy in patients, taking into account some of their phenotypic characteristics.Purpose: To study the relationship of the acetylator phenotype with the features of the clinical course of brucellosis in patients to develop prognostic criteria for the development of complications and indications for the use of antibiotics depending on the degree of N acetyltransferase activity.
2. Materials and Methods
- The study included 178 patients with various clinical forms of brucellosis. The age of the patients ranged from 18 to 46 years. Among the examined patients, men and women accounted for 99 (56%) and 79 (44%).The acetylation phenotype was determined in urine by the method of A.M. Timofeeva modified by G.A. Ponomarev (2017). Sulfadimezin was used as a test drug. Sulfadimezin (SD) has several features: rapid and complete absorption in the gastrointestinal tract, insignificant binding to tissue and plasma proteins, low level of liver extraction, complete conversion in the liver, and insignificant elimination by the kidneys in the unchanged form [3,5]. It is known from the literature that the biotransformation of SD in the body is carried out in the liver and leads to the formation of 2 main metabolites - free sulfadimezine and acetylated sulfadimezine (N-ACSD). The first stage of metabolism is carried out by N-demethylation of SD Mellitus. Further, free sulfadimesin as a foreign amine undergoes a biological acetylation reaction through an intermediate with coenzyme A and the formation of an acetyl conjugate - N-ACSD (second stage). The SD load was carried out based on 6 mg/kg of the mass of the studied organism, and then the demethylating ability was assessed, respectively, by the ratio of the amount of free sulfadimezine and acetylated sulfadimezine (N-ACSD) in the urine to the amount of sulfadimesin administered. The content of metabolites in urine was determined by a colorimetric method based on the formation of a red indophenol type compound with phenol in an alkaline medium in the presence of potassium ferricyanide [8,9]. Sulfadimezin was given in the morning on an empty stomach with a glass of boiled water or weak tea, fruit juice, milk, sour milk; the urine excreted after 7 hours was collected in a clean dish. After 1 hour of exercise, food could be eaten. To determine the main metabolites of CA, the following reagents are required: 1) 0.02% phenol solution; 2) 1% solution of red blood salt; 3) ammonia buffer (pH 10.50-10.60); 4) 12.5% trichloroacetic acid solution; 5) 36% concentrated hydrochloric acid specific gravity 1.17-1.19. And determined the content of acetylated and non-acetylated SD to the total amount of the drug, assessed the activity of acetylation.Equipment. Water bath; a rack with test tubes; foil-wrapped corks; pipettes with a capacity of 1 and 5 ml; FEC.Material. Urine-containing free and acetylated sulfonamides. The collected urine is brought to a volume of 25 ml with distilled water and filtered through a paper filter. The method is based on the ability of diazotized sulfanilamide, when interacting with acetylated H-acid (1,6-aminooxynaphthalene-3,5-disulfonic acid), to form a pink complex, the intensity of which is proportional to the concentration of sulfanilamide The content of the sum of sulfonamides (acetylated and free) in urine is established after hydrolysis of samples with hydrochloric acid, during which the free form of sulfonamide is formed from the acetylated one. Determination progress. In two test tubes (one for determining the total, and the other for free sulfadiazine) measure 1 ml of urine diluted 20 times, 1.5 ml of distilled water, and 0.25 ml of hydrochloric acid solution. In the third test tube (control) add 2.5 ml of water and 0.25 ml of hydrochloric acid solution. One tube (for the determination of total sulfadimezin) is closed with a foil-wrapped stopper and placed for hydrolysis in a boiling water bath for 15 minutes. The tube is then cooled.Pour 2 drops of sodium nitrite solution into all three test tubes, mix the contents thoroughly and leave to stand for 10 minutes. Add 1.5 ml of saturated sodium acetate solution to all samples, close the tubes with stoppers and shake them vigorously several times. Add 0.25 ml of acetylated H-acid solution to all tubes. Mix the contents again and leave the samples to stand for 15 minutes to allow color development.The extinction of the experimental samples is measured against the control on FEC e at 440 nm (blue filter) in a cuvette with a layer thickness of 0.5 cm. The calculation is carried out according to the formula
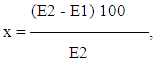 where x is the acetylating ability of the organism, % of acetylated sulfadimezinE1 - extinction of free sulfadimezine contained in the sample that has not undergone hydrolysis;E2 - extinction of total sulfadimezine (free + acetylated) contained in the sample subjected to hydrolysis.The indicator below 50% corresponds to the MA phenotype, 50%, and above to the BA phenotype.Immunological studies were carried out in the laboratory of chronic infectious process (head of the laboratory - Doctor of Medicine, Professor N.G. Gulyamov) RIEMID MH RUZ (Research Institute of Epidemiology, Microbiology and Infectious Diseases Ministry of Health of the Republic of Uzbekistan) (Deputy Director - Professor M.D. Akhmedova).For the quantitative registration of antigen-binding lymphocytes (ABL), specifically sensitized against tissue antigens (TA) of various organs, the reaction of indirect rosette formation (RIRF) was used according to the method of Garib F. Yu. (1981) [19]. A suspension of lymphocytes (0.1 ml 2x106 cells / ml) is mixed with 0.1 ml of antigenic (TA) erythrocyte diagnostic. The mixture is incubated for 1 hour at 40C. The resulting rosettes are fixed by adding 0.25% glutaraldehyde solution. The mixture is centrifuged and smears are obtained from the sediment on a glass slide, the smear is stained with Romanovsky-Giemsa paint. The percentage of rosette-forming lymphocytes is counted on smears under the immersion system of the microscope. The content of ABL in the blood of patients is determined by the difference in indicators between rosette formation with TA and serum albumin.As you know, when analyzing the results of immunological studies in patients, it is advisable to consider and analyze the direction and severity of changes in indicators in comparison with those in healthy individuals, when studying the dynamics of changes – in comparison with the previous period of the disease, and when assessing the effectiveness of treatment – in comparison with indicators before treatment [16,17].Proceeding from this, in the study of immunological indicators and their dynamics, we used the induction index - ↑ II, which reflects the direction and severity (multiplicity) of changes in the indicator upward, as well as the suppression index - ↓ IS - reflecting the direction and severity (multiplicity) of changes indicator downward. The use of ↑ II or ↓ IS allows an objective comparative assessment of one's results with the studies of other researchers in a specific pathology, as well as in the process of treatment.
where x is the acetylating ability of the organism, % of acetylated sulfadimezinE1 - extinction of free sulfadimezine contained in the sample that has not undergone hydrolysis;E2 - extinction of total sulfadimezine (free + acetylated) contained in the sample subjected to hydrolysis.The indicator below 50% corresponds to the MA phenotype, 50%, and above to the BA phenotype.Immunological studies were carried out in the laboratory of chronic infectious process (head of the laboratory - Doctor of Medicine, Professor N.G. Gulyamov) RIEMID MH RUZ (Research Institute of Epidemiology, Microbiology and Infectious Diseases Ministry of Health of the Republic of Uzbekistan) (Deputy Director - Professor M.D. Akhmedova).For the quantitative registration of antigen-binding lymphocytes (ABL), specifically sensitized against tissue antigens (TA) of various organs, the reaction of indirect rosette formation (RIRF) was used according to the method of Garib F. Yu. (1981) [19]. A suspension of lymphocytes (0.1 ml 2x106 cells / ml) is mixed with 0.1 ml of antigenic (TA) erythrocyte diagnostic. The mixture is incubated for 1 hour at 40C. The resulting rosettes are fixed by adding 0.25% glutaraldehyde solution. The mixture is centrifuged and smears are obtained from the sediment on a glass slide, the smear is stained with Romanovsky-Giemsa paint. The percentage of rosette-forming lymphocytes is counted on smears under the immersion system of the microscope. The content of ABL in the blood of patients is determined by the difference in indicators between rosette formation with TA and serum albumin.As you know, when analyzing the results of immunological studies in patients, it is advisable to consider and analyze the direction and severity of changes in indicators in comparison with those in healthy individuals, when studying the dynamics of changes – in comparison with the previous period of the disease, and when assessing the effectiveness of treatment – in comparison with indicators before treatment [16,17].Proceeding from this, in the study of immunological indicators and their dynamics, we used the induction index - ↑ II, which reflects the direction and severity (multiplicity) of changes in the indicator upward, as well as the suppression index - ↓ IS - reflecting the direction and severity (multiplicity) of changes indicator downward. The use of ↑ II or ↓ IS allows an objective comparative assessment of one's results with the studies of other researchers in a specific pathology, as well as in the process of treatment.3. Research Results and Their Discussion
- According to the results of the study, it was revealed that of 178 patients, 102 (57.3 ± 3.7%) had a fast type of acetylation (FA), 76 (42.7 ± 3.7%) had a slow type of acetylation (SA). To determine the intermediate type of acetylation, we conducted a test in patients before discharge, and compared the results of both tests; in the examined patients with brucellosis, no intermediate type of acetylation was revealed.The frequency of occurrence of various acetylator phenotypes was characterized by the predominance of FA, which may indicate their predisposition to brucellosis.When analyzing the sex and age of patients depending on the type of PHA, it was revealed that 51.0% of patients with FA were males and 49.0% were females (P> 0.05). The average age of FA patients was 39.3 ± 0.44, in SA the average age was 38.7 ± 0.53 (P> 0.05). As can be seen from the studies presented, no significant differences were found between the groups. This is consistent with the literature studies that PHA does not depend on age and sex [21].The analysis revealed the dependence of the form of brucellosis on the type of PHA. Thus, in patients with FA, there was mainly acute - 43 (42.2 ± 4.9%) and subacute - 34 (33.3 ± 4.7%) forms of brucellosis, in SA the acute and subacute forms of brucellosis, respectively, were 5, 3 ± 2.6% (4 patients) and 7.9 ± 2.7% (6 patients) and these differences were statistically significant (P< 0.01). For patients with SA, primary - chronic 17 (22.4 ± 4.8%) and secondary chronic - 49 (64.5 ± 5.5%) forms of brucellosis were more typical. In patients with primary and secondary chronic forms of brucellosis, FA occurred in 7 cases, respectively - 6.9 ± 2.5% and 18 - 17.6 ± 3.7% cases, respectively, which is significantly less compared with SA (P< 0.01).Based on the presented studies, a relationship has been established between the PHA rate and various forms of brucellosis. For acute forms of brucellosis, a fast type of PHA is characteristic, for chronic forms of brucellosis, a slow type.The severity of brucellosis also depends on the type of PHA. In patients, brucellosis mainly proceeded in a moderately severe form - 107 (60.0 ± 3.7%), in 49 patients - 27.5 ± 3.3% of cases - in a mild one and in 12.4 ± 2.5% (22 patients) - severe. In FA, brucellosis was mainly mild - 36 patients (35.3 ± 4.8%) and moderately severe - 63 patients (61.8 ± 4.9%), only in 3 cases (2.9 ± 2.1%) there was a severe course of the disease.In SA, the disease proceeded mainly in severe - 19 (25.0 ± 4.8%) and moderate - 44 (57.9 ± 5.6%) forms, only in 13 (17.1 ± 4.3%) cases it was stated easy course of brucellosis.Slow type. In patients with SA, the disease lasts longer with splenomegaly, lymphadenopathy, and joint damage. The incidence of the main clinical symptoms of brucellosis did not depend on the type of PHA, but in SA the duration of the main symptoms of the disease was longer than in FA.Further, we studied the indicators of ABL to tissue and the rate of acetylation, and various forms of brucellosis. Acute forms of brucellosis are characterized by a rapid type of PHA, for chronic forms of brucellosis antigens of joints, myocardium, liver, and brain in patients with brucellosis with different acetylator activity. According to F. Yu. Garib content of ABL to tissue antigens of the organ up to 2% or less is not an indicator of the presence of any pathological process in the tissue of this organ [6,10].In patients with brucellosis and FA, the content of ABL to liver TA was 2.8 ± 0.13%, where the multiplicity of increase in comparison with the upper limit of the norm was - ↑ II = ↑ 1.6 times, ABL to myocardial TA was 2.1 ± 0, 17% (↑ II = ↑ 1.1 times). The content of ABL to TA of the joints was 4.7 ± 0.23% (↑ II = ↑ 2.4 times), and ABL to TA of the brain was 2.5 ± 0.15% (↑ II = ↑ 1.3 times). Comparative analysis of ABL indicators to TA of joints in patients with FA and SA showed that in SA the joint damage was almost 2 times more intense (P< 0.01) compared to FA, this is an indicator of the development of pronounced inflammation processes that cause destruction and necrosis cells in the tissue of joints in patients with SA. It was noted that the main source of endogenous intoxication in patients in this group is the joint tissue. In patients with SA, ABL to TA of the liver, myocardium, and brain were higher compared with FA, but these differences were not significant (P> 0.05).As It is known from the literature, acetylation processes occur mainly in the system of mononuclear phagocytes; Kupffer cells of the liver, macrophages of the spleen, intestinal lymph nodes, they are most intense in the lymph nodes [1,2,6,7], and in SA the inflammatory process is sluggish (Khusainova V. Kh., 2003), in this regard, in our patients with SA, the inflammatory process lasted longer and the transition to a chronic process was more frequent, compared with FA [5,9].The same degree of increase in ABL to TA of the liver, myocardium, and brain in patients, regardless of the type of PHA, indicates that they have pronounced processes of inflammation, destruction, or necrosis of cells and all organs are equally a source of endogenous intoxication of the body (1- drawing). Against the background of a high degree of endogenous intoxication, an increase in ABL to TA of the brain is noted, which makes it possible to judge the failure of the blood-brain barrier to maintain homeostasis in the brain tissue.Despite the rather high rates of ABL to TA of the liver, brain, and myocardium, there were no clear signs of hepatitis, myocarditis, and encephalitis in patients with SA. This allows us to judge that, against the background of the destructive processes taking place, the intact part of the organ tissue compensates for the function of the damaged tissue [15].
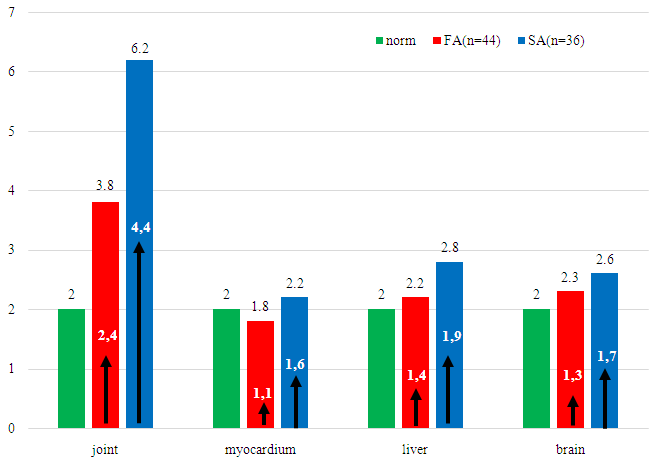 | Figure 1. ABL index to TA of some organs depending on the type of PHA (n = 80) |
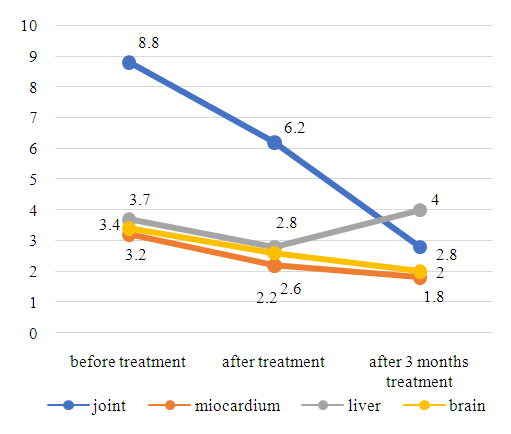 | Figure 2. ABL index to TA of some organs in patients with SA in dynamics (n = 36) |
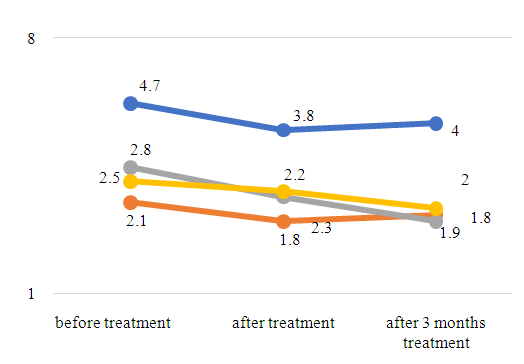 | Figure 3. ABL index to TA of some organs in FA patients over time (n = 44) |
4. Conclusions
- Obligatory determination of the type of acetylation phenotype: in cases of detection of a slow type of acetylation (SA), the appointment of combined antibiotic therapy should be controlled depending on the value of ABL to the TA of the joints with cancellation or continuation of treatment and add hepatoprotectors to prevent hepatotoxic effect and detoxification therapy; in cases of detection of a rapid type of acetylation (FA), the appointment of a longer course of combined antibiotic therapy, depending on the value of ABL to TA of the joints, cancel or continue treatment.In our work, we have shown that the use of drugs, considering their metabolism, leads to an increase in the quality of treatment for brucellosis in patients. The obtained results of the relationship between the clinical manifestations of brucellosis and the effectiveness of its pharmacotherapy, considering the activity of N acetylation, make it possible to develop new approaches to individualized therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML