-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(6): 459-464
doi:10.5923/j.ajmms.20211106.01
Received: May 12, 2021; Accepted: May 31, 2021; Published: Jun. 15, 2021

Improvement of the Diagnosis and Treatment of Allergic Rhinitis in Patients with Bronchial Asthma (Molecular Genetic Analysis)
Fotima Sharipovna Iskhakova, Nilufar Jurakulovna Khushvakova, Gulrux Bakhtiyorovna Davronova, Abdurasul Abdukarimovich Khamidov
Samarkand State Medical Institute, Uzbekistan
Correspondence to: Fotima Sharipovna Iskhakova, Samarkand State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this article, the improvement of the diagnosis and treatment of allergic rhinitis in patients with bronchial asthma (molecular genetic analysis) is highlighted. It is also scientifically proven that the patient has difficulty breathing during an attack, chest tightness, swelling of the jugular veins, swelling of the face, dilation of the nostrils. In patients with bronchial asthma, a wheezing sound and wheezing are described. In addition, conclusions and recommendations for improving the diagnosis and treatment of allergic rhinitis in patients with bronchial asthma (molecular genetic analysis) are presented.
Keywords: Diagnosis of bronchial asthma, Allergic rhinitis, Seizures, Wheezing, Swelling of the jugular veins, Chest pain
Cite this paper: Fotima Sharipovna Iskhakova, Nilufar Jurakulovna Khushvakova, Gulrux Bakhtiyorovna Davronova, Abdurasul Abdukarimovich Khamidov, Improvement of the Diagnosis and Treatment of Allergic Rhinitis in Patients with Bronchial Asthma (Molecular Genetic Analysis), American Journal of Medicine and Medical Sciences, Vol. 11 No. 6, 2021, pp. 459-464. doi: 10.5923/j.ajmms.20211106.01.
1. Introduction
- Bronchial asthma is a chronic inflammatory disease of the respiratory tract, characterized by the following symptoms: complete or partial re-obstruction of the airways, spasm of bronchial smooth muscle, mucosal edema, infiltration of the subcutaneous layer with inflammatory cells, mucosal hypersecretion, thickening of the basement membrane; episodes of cough, wheezing, wheezing, chest tightness, which occur under the influence of special trigger factors and occur mainly in the evening or morning; respiratory hyperreactivity. Bronchial asthma is one of the most common chronic diseases in childhood. in the structure of recurrent bronchopulmonary pathology, asthma accounts for 50-60% [1]. Epidemiological data show that 5-20% of children are infected with BA, according to ISAAC (International Organic Organization for Asthma and Allergy in Children). In many hands, the external disease is put on 2-6 years after the onset of the disease, which worsens the consequences of the disease.In the etiology of bronchial asthma, two groups of factors are distinguished: antigenic and noantigenic. Antigenic factors include exogenous allergens, among which non-infectious allergens play an important role.- "domestic" allergens (dust, epidermal, insects and insects, aquarium fish food) [2]:Ø pollen allergensØ food allergensØ drugs, including gamma globulin and protein drugs.There are clear laws of the formation of noninfectious sensitization in children. Allergodermatosis is a common form of food allergy at an early age. In some patients, the target organ in early food sensitization is the digestive tract and respiratory system. The onset of epidermal sensitization (animal hair, feather pillows, etc.) often begins concurrently with respiratory allergies, at which stage respiratory allergies occur with rhinopharyngitis, laryngotracheitis, and “minor” forms of respiratory allergies. Respiratory allergic diseases are sometimes mistakenly diagnosed as an infectious disease, which in turn leads to the use of multiple drugs and the development of drug allergies. In many children, the typical typical clinical picture of bronchial asthma occurs after the addition of sensitization to house dust, and its formation occurs by the age of 2-5 years of life. In children, sensitization to plant flowers is formed in adulthood, sensitization to epidermis and house dust allergens can be observed at the age of 2-5 years [3].Asthma is also a heterogeneous disorder, and as Wenzel suggested, definitions of asthma are problematic because of its complexity, possibly reflecting a collection of different phenotypes. Most clinical definitions elaborate on the symptoms (eg, wheezing and difficulty breathing) of lung functions, of exacerbations and, often, of the response to medication (eg, high-dose corticosteroids). It is estimated that asthma affects 4% to 11% of the general population. Evidence in Canada also suggests that the symptoms of patients with asthma continue to be inadequately controlled according to guidelines [4].In the literature, there is increasing recognition of a link between asthma and AR. It should be noted, however, that there are other patients: those with nonatopic asthma and those with non-AR. Although these disorders are beyond the scope of the present review, it is recognized that many patients with nonallergic asthma have non-AR and/or sinusitis. The link between these problems may not be as close as the link between allergic asthma and AR; nevertheless, it has been supported by some studies. Simons reviewed some of the early concepts linking AR and asthma, and suggested that the connection between the two had actually been known for centuries, but that specialization in medicine, as well as in medications, led to the perspective of separate disease entities. To reconnect asthma and AR, Simons proposed using the term ‘allergic rhinobronchitis’. Another current concept is ‘one airway, one disease’, which was originally suggested by Grossman in 1997 [5]. It seems logical that an uninterrupted air passage from the nose to the alveolar ducts of the lungs would have many similarities. The following discussion elaborates on the connections between AR and asthma in terms of their epidemiology, pathophysiology and similar responses to treatment. Each of these disorders has a variety of effective treatment modalities; asthma responds well to inhaled corticosteroids and beta2-agonists, as well as to a new adjunctive therapy approved in Canada, the anti-immunoglobulin E (anti-IgE) agent omalizumab. Treatment for AR usually involves various forms of antihistamines and decongestants, as well as glucocorticosteroids. The focus of the present review is on a treatment that is effective for both disorders, in particular, the leukotriene receptor antagonists (LTRAs) [6].Among the most frequently encountered comorbid conditions associated to asthma are rhinosinusitis, gastro-oesophageal reflux disease (GERD), psychological disturbances, chronic infections and obstructive sleep apnoea (OSA). Various other conditions and contributing factors that may influence asthma control are shown on (Figure 1) [7].
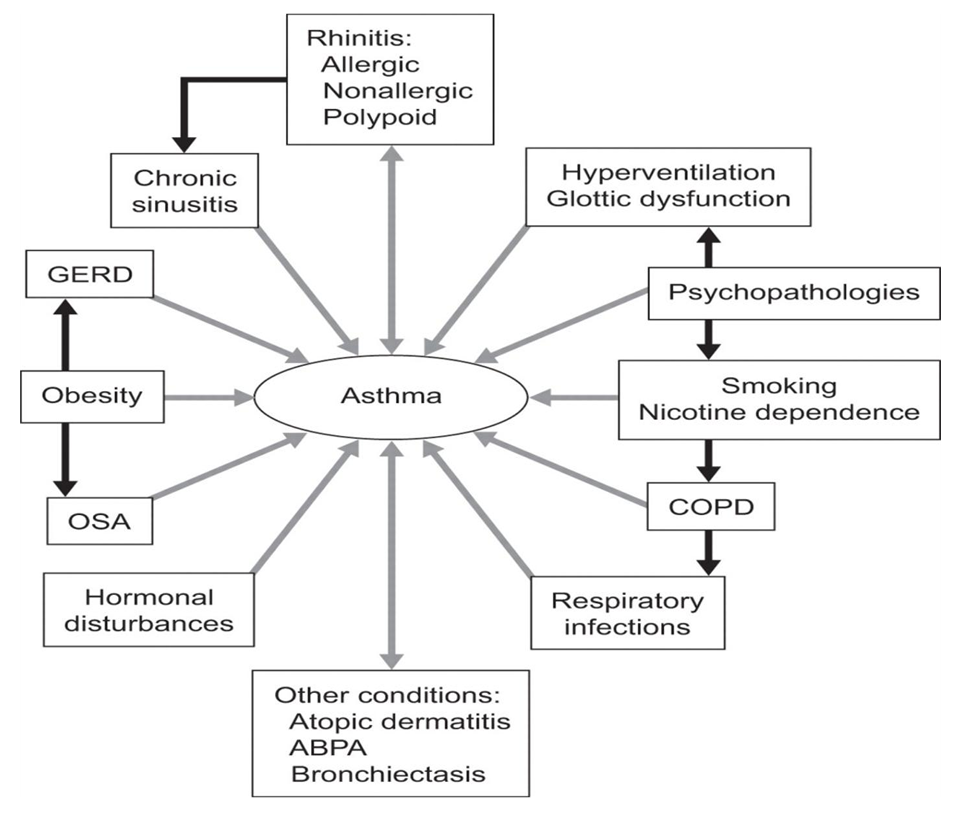 | Figure 1. Respiratory joint diseases |
2. Materials and Methods
- Heredity [8]:1. If the child's parents do not have atopy, the risk of developing the disease in the child is 0-20%.2. If the symptoms of atopy are present only in the father, the child develops atopic symptoms in 33% and asthma in 25%.3. If the symptoms of atopy are present only in the mother, then atopy occurs in 48% of siblings and asthma in 38% of children.4. If the father and mother have symptoms of atopy, then the risk of developing atopy and bronchial asthma is 60-100%.Hypersensitivity to environmental allergens, which is ignored by the immune system of a healthy person, is inherited. In cases of hereditary predisposition to atopic diseases, BA begins at an early age and is severe. The most common cause of BA in young children is an acute respiratory viral infection with a strong sensitizing effect on the body, which affects the respiratory tract. increases the permeability of the mucous membranes to various aeroallergens, while the antigenic properties of viruses call for immunological remodeling of the macroorganism during the infectious process. In young children, for a long time, BA is replaced by "ORVI with obstructive syndrome", "recurrent obstructive bronchitis" and irrational treatment is prescribed. Many years later, the child is diagnosed with typical bronchial asthma [9]. We have shown that after antigen challenge, there is an increase in IL-5-producing T cells in the bone marrow and an increase in high-affinity IL-5 receptor, which is associated with an elevated number of eosinophil progenitors. Recently, we showed that this process is most likely due to retrograde migration of antigen-specific T cells from the airways to the bone marrow, where antigen-specific T cells can produce a number of cytokines and help to release and differentiate the progenitor cells. Progenitor cells can be found along the entire airway in atopic individuals and can differentiate into mature eosinophils in response to local antigen challenge Figure 2) [10].
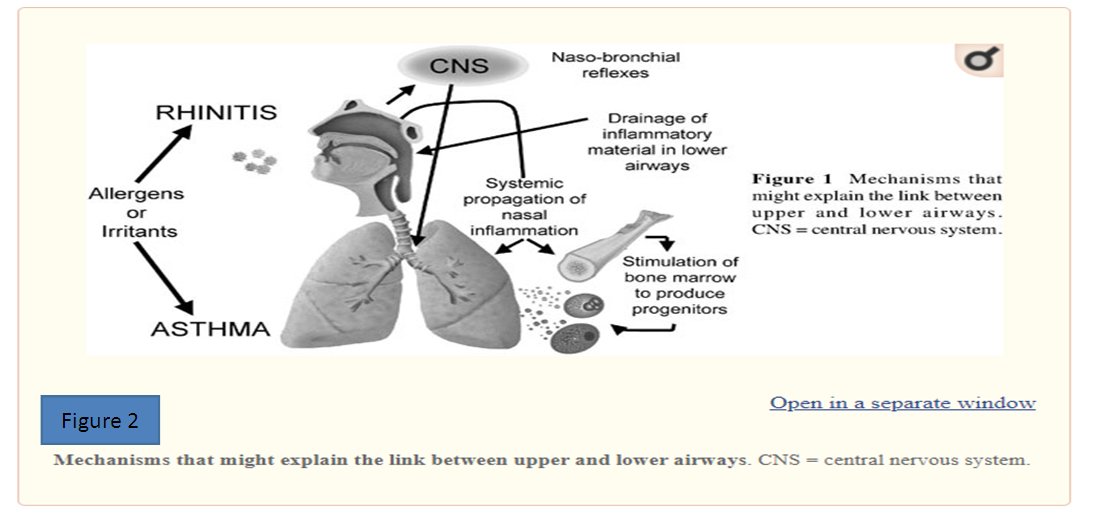 | Figure 2 |
3. Results
- Greiff and colleagues treated nonasthmatic allergic rhinitis patients with inhaled corticosteroids during pollen season. They found an inhibition of the increase of eosinophils in blood and nasal tissues that is usually observed in pollen season. The patients who received inhaled budesonide had significantly milder nasal symptoms. In a recent clinical study, asthmatic individuals with nasal polyposis treated with montelukast had a 70% improvement of nasal symptoms and a 60% to 90% improvement in asthma clinical score. In a study comparing treatment with montelukast alone to treatment with inhaled and intranasal corticosteroids in patients with allergic rhinitis and in patients with asthma, only the group treated with corticosteroids showed a significant reduction in nasal nitric oxide and in nasal peak flow, whereas both treatments were efficient in decreasing rhinitis symptoms [13]. Immunotherapy is reserved for patients with moderately severe allergic rhinitis. Immunotherapy reduces inflammatory-cell recruitment and activation as well as the secretion of mediators. In a group of allergic rhinitis patients with asthma, immunotherapy improved methacholine hyperreactivity and quality of life and reduced seasonal asthma symptoms. Reducing the allergen sensitivity not only leads to relief of rhinitis but also helps control asthma (although less effectively). When collecting a medical history, the following should be considered.- Presence of atopic dermatitis, allergic rhinoconjunctivitis, bronchial asthma or other atopic diseases in the family.Presence of one of the following symptoms [14]:- cough, intensified in the evening;- repeated whistling;- repeated episodes of difficulty breathing;- feeling of tightness in the chest.Occurrence or worsening of symptoms:- at night;- in contact with animals;- with chemical aerosols;- house dust;- flower pollination;- tobacco smoke;- when the ambient temperature drops;- when taking aspirin, adrenoblockers;- after physical exertion;- ORVI;- after strong emotional tension;
4. Discussion
- There are several conditions that may mimic asthma, such as hyperventilation syndrome or various types of glottic dysfunction, and they usually present as a paradoxical adduction of the vocal cords during inspiration [15]. These conditions are also often associated with anxiety or other psychological disturbances. Newman et al. reported that 56% of 95 patients fulfilling the criteria of paradoxical vocal cord motion disorder, proven with laryngoscopy, had a concomitant asthma. Hussein et al. reported that paradoxical vocal cord dysfunction is more common among females and older individuals, and can be a comorbidity associated with asthma, GERD and previous abuse. Psychotherapy and/or speech therapy may address the problem in such cases [16]. It is unknown whether comorbid asthma and hyperventilation syndrome or glottic dysfunction alters the long-term clinical expression of asthma. Worldwide, obesity is at epidemic levels, and there is significant morbidity associated with it. Obesity is associated with an increased prevalence of asthma, particularly among the morbidly obese and females, and a causal relationship between obesity and asthma has been suggested by recent animal and human studies. Furthermore, all studies evaluating the effects of weight loss in the obese have shown an improvement in asthma symptoms, control and medication needs. Mechanical, inflammatory and genetic/developmental factors and a higher prevalence of comorbidities have been implicated in the development of asthma in obese individuals [17].Obesity has a major role in the development of OSA and GERD, and may theoretically act on asthma through these associated conditions. Mosen et al. have suggested, however, that even after adjusting for demographics, smoking status, oral corticosteroid use and evidence of GERD, obese adults were more likely than those with a normal body mass index (BMI) to report poor asthma-specific quality of life, poor asthma control and a history of asthma-related hospitalisations. There has been a parallel increase in prevalence of obesity, OSA and asthma in the past years. The possible causal association of asthma and OSA has not, however, been systematically studied [18].Asthma in the obese patient appears to be a specific phenotype associated with pulmonary function changes caused by breathing at low lung volumes, a systemic inflammatory process that may possibly influence airways and a reduced response to asthma medications. Thus, all of these factors combined probably explain why asthma is difficult to control in these individuals.
5. Conclusions
- Numerous comorbidities are frequently associated with asthma and may influence the clinical expression and severity of asthma. They should be investigated and treated appropriately in order to determine their respective influence on asthma and improve asthma control. More research is needed to shed further light on the relationships between the various common comorbidities associated with asthma and the clinical features and outcomes of those suffering from asthma. AR and asthma often respond to the same treatments, which suggests that there is an intimate connection between the two. As a result, treatment guidelines have recognized the link between AR and asthma, and recommended that each condition be evaluated when a patient presents with either of the disorders. The guidelines further support a combined approach to treating both conditions for maximum therapy and a decreased medication load [19]. Future guidelines may even consider recommending that treatment be aimed for total or overall respiratory symptom control. This approach would address both upper and lower airway symptoms in a more comprehensive manner.In summary, the search for treatments to improve the symptoms of patients afflicted with AR and asthma has targeted the CysLTs, one group of the many inflammatory substances in the allergic response. Research has revealed that when the CysLTs are blocked, symptoms and function improve. Steroids and antihistamines do not block the action of leukotrienes, but the LTRA montelukast is an effective blocking agent. The evidence suggests that montelukast reduces the symptoms of AR, and is comparable with antihistamines and oral decongestants; it is also effective in both SAR and PAR, and improves lung functioning in asthma. In fact, results suggest that the effectiveness increases with greater levels of allergen exposure, and efficacy is also increased when asthma symptoms are more severe. As a result, montelukast is a suggested treatment for both conditions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML