-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(5): 398-401
doi:10.5923/j.ajmms.20211105.08
Received: Apr. 24, 2021; Accepted: May 11, 2021; Published: May 15, 2021

X-Ray and Tomographic Manifestations of Tuberculous Spondylitis
Babоev Abduvakhob Sakhibnazarovich
Bone and Joint Tuberculosis Department of Rebuplican Specialized Scientific Practical Medical Center of Phtiziology and Pulmonology, Tashkent, Uzbekistan
Correspondence to: Babоev Abduvakhob Sakhibnazarovich, Bone and Joint Tuberculosis Department of Rebuplican Specialized Scientific Practical Medical Center of Phtiziology and Pulmonology, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

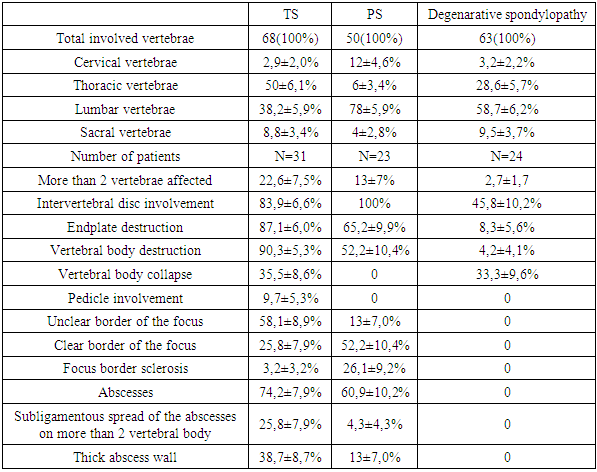
X-ray and tomographic data of seventy-eight patients with spine disorders (31 patients with tuberculous spondylitis, 23 patients with pyogenic spondylitis, and 24 patients with degenerative spondylopathy) after clinical, laboratory, bacteriologic, and histologic verification of diagnosis was comparatively analyzed. Current X-ray and tomographic features of tuberculous spondylitis are relatively equal thoracic and lumbosacral spine segments localization, with more than two adjacent vertebrae involvement in 22,6±7,5% of cases, the collapse of the vertebra(s) in 35.5 ± 8.6% of cases, unclear or osteoporotic border of bone tissue around TB focus in 64.5 ± 8.6%, formation of abscesses in 74.2±7.9% and their subligamentous spread to more than two vertebrae in 25,8±7,9% of cases, intact intervertebral disc in 16.1 ± 6.6% of cases, and specific process in the lungs in 48.4 ± 9.0% of cases.
Keywords: Tuberculous spondylitis, Pyogenic spondylitis, X-ray and tomographic features
Cite this paper: Babоev Abduvakhob Sakhibnazarovich, X-Ray and Tomographic Manifestations of Tuberculous Spondylitis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 5, 2021, pp. 398-401. doi: 10.5923/j.ajmms.20211105.08.
Article Outline
1. Introduction
- X-ray and tomographic (MRI and CT) methods of examination play a leading role in the diagnosis of tuberculous (TB) lesions of the spine [1,2,3].Features of radiological changes of spine TB depend on the activity and duration of the specific process [1,2].MRI is considered the most informative method of radiological diagnosis of tuberculous spondylitis (TS) in the current time [4]. MRI makes it possible to assess the size and location of abscesses, the length, and degree of compression of the spinal cord and its roots [4]. Yet, for analysis of bone tissue structure, CT is indispensable and provides better information about bone density at the border of destruction, presence of sequestration, and calcifications inside or at the border of the abscesses [5].However, despite the presence of X-ray, CT, and MRI the percentage of misdiagnosis of spinal TB is still high, and amounts up to 35% [3], which requires a search for additional criteria for differential diagnosis of TS.
2. Purpose
- Comparative analysis of X-ray, MRI, and CT features of patients with tuberculous spondylitis (TS), pyogenic spondylitis (PS), and degenerative spondylopathy (DS) to identify differential diagnostic features of tuberculous spondylitis (TS).
3. Materials and Methods
- The study was conducted at the bone and joint TB department of the Republican Specialized Scientific and Practical Medical Center of Phthisiology and Pulmonology of the Republic of Uzbekistan. Analysis of the results of a comprehensive examination of 78 (46 (59±5.6%) male and 32 (41±5.6%) female) patients aged from 17 to 81 years (mean age – 50 years), with spinal lesions, was performed. All patients underwent an x-ray of the affected segment of the spine and lungs, MRI, and CT studies. According to the bacteriological and histological results of operated patients, as well as clinical and laboratory finding the patients were divided into 31 patients with TS, 23 patients with PS, and 24 patients with degenerative (metabolic, autoimmune) lesions of the spine. After that, a comparative analysis of X-ray and tomographic signs was performed.
4. Results
- In 31 (100%) patients with TS, X-ray examinations were performed, in 20 (64.5±8.6%) cases, an MRI was performed, in 6(19.4±7.1%) cases, a CT was performed and in 4(12.9±6.0%) cases both CT and MRI studies were performed.In the TS group of patients, thoracic and lumbar spine lesions prevailed, involving 2 adjacent vertebrae. More than 2 vertebrae were affected in 7(22.6±7.5%) patients.Five (16.1±6.6%) patients had a specific process in the lumbosacral region. The lumbar spine localization of the specific process had 10(32.3±8.4%) patients. The thoracic spine was involved in 13(41,9±8.9%) cases. Only one (3.2 ± 3.2%) had cervical spine localization.The intervertebral disc was intact in 5(16.1±6.6%) cases of the TS group, in the remaining 26(83.9±6.6%) cases, there was a narrowing of the intervertebral space. No endplate destruction was observed in 4(12.9±6.0%) cases since the process was localized deep in the vertebral bodies and in 27(87.1±6.0%) cases, the endplates were destroyed. In 10(32.3±8.4%) cases, the vertebral bodies were destroyed superficially. Twenty (64.5±8.6%) patients had deep destruction of the vertebral bodies with the involvement of the pedicles of the vertebrae in 3(9.7±5.3%) cases. The collapse of one or several involved vertebrae was observed in 11(35.5±8.6%) patients, in 7 (22.6±7.5%) cases it led to kyphotic deformity of the spinal segment.Bone structure evaluation in the TS group showed sclerotic border of the focus in 1(3.2 ± 3.2%) case, clear border in 7(22.6±7.5%) cases, visually unclear border in 7(22.6±7.5%) cases, unclear border combined with osteoporosis of the spine segment in 13(41.9±8.9%) cases, and in 3(9.7±5.3%) cases the process was manifested only by osteoporosis of the involved vertebral bodies.Paravertebral abscesses were detected in 23(74.2±7.9%) cases of the TS group, in 12(38.7±8.7%) cases the abscess wall was thick, in 11(35.5±8.6%) cases it was thin, and in 1(3.2±3.2%) case there were calcifications in abscesses. Paravertebral soft tissue infiltration observed in 7(22.6±7.5%) cases and 1(3.2 ± 3.2%) patient had no soft tissue reaction. Subligamentous spread of the abscesses on more than 2 vertebral body observed in 8(25,8±7,9%) cases.Chest X-ray showed previous pulmonary TB in 10(32.3±8.4%) cases, pleurisy in 2(6,5±4,4%) cases, and active pulmonary TB in 4(12.9±6.0%) cases.All 23(100%) PS group patients underwent an MRI and both MRI and CT were performed in 5(21.7±8.6%) cases. Two adjacent vertebrae were affected in 20(87±7.0%) cases, and 19(82.6±7.9%) patients had the lumbar spine localization of the inflammatory process. Intervertebral disc was affected in all 23(100%) cases. More than 2 vertebrae were affected in 3(13.0±7.0%) cases of the PS group. Endplates destruction was observed in 15(65.2±9.9%) cases, 8(34.8±9,9%) of those cases were presented with deep vertebral body destruction. The kyphotic deformity was observed in 3(13±7.0%) cases. The collapse of the vertebral body or involvement of pedicles of the vertebra was not observed in the entire group. Thus, in the PS group, more than half of the patients (13(56.5±10.3%)) showed no major destruction of the vertebral bodies.Thick sclerotic bone reaction around the inflammatory focus was detected in 6(26.1±9.2%) patients of the PS group, 2(8.7±5.8%) of them had systemic osteoporosis. Sclerotic bone reaction detected in 9(39.1±10.2%) patients, 3(13±7.0%) of them had systemic osteoporosis. Systemic osteoporosis without bony destruction was detected in 2(8.7±5.8%) patients. No sclerotic border of the bone around the inflammatory focus was detected in 3(13±7%) patients and one of them had systemic osteoporosis. Thus, signs of bone tissue sclerotic reaction were present in 18(78.3±8.6%) patients with pyogenic spondylitis.Paravertebral soft tissues were infiltrated in 9(39.1±10.1%) patients, abscesses formation was detected in 14(60.9±10.2%) patients. Thick abscess wall visualized in 3(13±7.0%) cases and thin abscess wall in 11(47.8±10.4%) cases. Subligamentous spread of the abscesses on more than 2 vertebral bodies observed in 1(4,3±4,3%) case. Chest X-ray showed previous pulmonary TB in 2(8.7±5.9%) cases of the PS group.In the group of patients with degenerative spondylopathy 16(66.7± 9.6%) patients underwent MRI, 2(8.3±5.6%) patients underwent CT, and 6(25±8.8%) patients underwent both examinations. One (4.2 ± 4.1%) patient had a lumbar plexus neuroma mimicking a psoas abscess. Osteoporotic vertebral compression fracture of several or one vertebral bodies was observed in 6(25±8.8%) patients. Ankylosing spondylitis with multiple lesions of the vertebrae, thickening of the anterior longitudinal ligament and bone marrow edema of the vertebral body angels observed in 6(25±8.8%) patients. Herniated intervertebral discs with degeneration and local edema of bone tissue due to aseptic inflammation of the surrounding bone tissue were 13(54.2±10.1%) patients. One (4.2±4.1%) patient with long-term family contact with a patient with pulmonary tuberculosis, positive tuberculin skin tests, and high levels of acute-phase blood proteins, who complained of pain in the lumbar spine, was found to have herniated intervertebral disc of VL4-5, VL5-S1 without signs of inflammation.
|
5. Discussion
- At the first stage of the diagnosis of tuberculous spondylitis, an orthopedic and neurological examination is performed, followed by an X-ray and tomographic examination of the affected segment of the spine [11]. X-ray and tomographic features of tuberculous spondylitis depend on the activity and duration of the tuberculous inflammation [1,2]. Tuberculous spondylitis in the active phase most often has to be differentiated from pyogenic spondylitis [12,13,14,15,16].MRI has great capabilities because it allows detecting infiltration of bone marrow and soft tissues, assessing intervertebral discs and spinal cord, which is unavailable for other techniques. This makes MRI a method of choice for diagnosing spinal diseases, including TS [1,2,3,12].According to the current literature, the distinctive features of TS on MRI are paravertebral abscesses, multiple vertebral involvement, well subligamentous abscess spread [6,7,10], destruction of terminal plates [8], vertebral destruction [14]. DJ Kotzé et al., 2006 studied MRI of histologically confirmed patients with TS and indicated that thickened walls of abscesses were present in 60% [6]. Na-Young Jung et al., 2004, having studied MRI of 52 patients with confirmed diagnoses of pyogenic or tuberculous spondylitis, concluded that thin and smooth abscess wall is characteristic of tuberculous spondylitis [7]. Ahmed Yehya et al., 2010 retrospectively studied MRI images of 30 patients who had confirmed spondylitis either tuberculous or pyogenic also concluded that a thin and smooth abscess wall was more suggestive of tuberculous spondylitis than pyogenic spondylitis [10].In our study, abscesses formation, abscess wall thickness, and subligamentous spread of the abscesses were comparatively equal in TB and PS patients, but the subligamentous spread of the abscesses on more than 2 vertebral body was observed in the TS group more often, than in PS group (25,8±7,9% of cases and 4,3±4,3% of cases, respectively).Chung-Jong Kim et al, 2016 performed a retrospective review of the medical records of 71 patients with culture-negative pyogenic spondylitis (CNPS) and 94 patients with TS and concluded that in both groups, the lumbar spine was the site most frequently affected, with lumbar involvement in 90.1% of the CNPS patients and 62.8% of the TS patients. The thoracic spine was involved more frequently in the TS group than the CNPS group (11.3 vs. 42.6%). Psoas and paravertebral abscesses were significantly more prevalent in patients with TS, whereas epidural abscesses were equally common in the two groups [9].In our study, the thoracic spine and lumbosacral region were involved in the tuberculous process equally in 48.4±9% of cases. Alternatively, PS was localized mostly (65.2±9.9%) in the lumbar spine. The high rate of thoracic spine localization of the TB process results from hematogenic dissemination of mycobacterium tuberculosis from the lungs, which is known to be the primary focus of TB infection. Both TS and PS groups were presented with two adjacent vertebrae lesions in our study. However, multiple vertebral body involvement was more common in the TS group, than in the PS group (in 22,6±7,5% cases and 13±7% cases, respectively). In addition, deep destruction of the vertebral bodies was observed in 64.5±8.6% cases of the TS group and only in 34.8±9,9% cases of the PS group. One or several involved vertebrae collapse was observed in 35.5±8.6% of cases with TS and the intervertebral disc was intact 16.1±6.6% cases, whereas all PS cases had intervertebral disc involvement and no vertebral body collapse was observed.Thus, the pathologic process originates in the vertebral body and expands to the intervertebral disc in TS; but originates from the intervertebral disc, then involving bone tissue of the vertebral body in PS.Many degenerative and inflammatory spinal disorders can mimic infectious spondylitis as it can have endplate bone marrow edema. Acute osteoporotic spinal fractures leading to bone marrow edema can mimic infectious spondylitis too [14]. On the other hand, tuberculous destruction located deep inside the vertebral body (as it was in 1(3,2±3,2%) case of our case series) can manifest with compression fracture of the vertebral body with no abscess formation [17]. Po-Ju Lai et al, 2017 reported 9 cases of tuberculous spondylitis after vertebroplasty [17] and suggested that patients with a history of pulmonary TB or any elevation of infection parameters should be reviewed carefully to avoid infective complications.In our case series, chest X-ray showed 48.4±9.0% of cases with active or previous pulmonary TB. Besides, bone structure analyses showed the sclerotic reaction of bone tissue around the inflammation focus in PS was present in 78.3±8.6% of cases, then in TS only in 25.8±7.9% of cases, so in PS sclerotic reaction was more common.
6. Conclusions
- Chest X-ray, involved spine segment MRI and CT should be performed for differential diagnosis of TS. Thoracic and lumbar spine localization of TS is equally common. More than two adjacent vertebrae involvement, deep destruction of the vertebral bodies, the collapse of the vertebral body, unclear or osteoporotic border of bone tissue around TB focus, formation of abscesses and their subligamentous spread to more than two vertebral body and signs of active or previous pulmonary TB are more suggestive of tuberculous spondylitis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML