-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(5): 362-366
doi:10.5923/j.ajmms.20211105.02
Received: Apr. 7, 2021; Accepted: Apr. 23, 2021; Published: Apr. 30, 2021

Efficacy and Safety of New Myocardial Stabilizer for Off-Pump Coronary Artery Bypass Grafting
Abdurakhmanov A. A.
Department of Cardiac Surgery, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Correspondence to: Abdurakhmanov A. A., Department of Cardiac Surgery, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan.
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the newly developed myocardial stabilizer in an experimental model in dogs. Introduction. Off-pump Coronary artery bypass grafting is still a technically challenging procedure. It has been demonstrated that mechanical stabilizers used to locally restrict cardiac excursion exhibit significant residual mobility that can lead to difficulty in performing the surgical procedure. Materials and methods. The study included 4 mongrel dogs weighing 12 to 20 kg. Surgical intervention was performed through theleft thoracotomy using a myocardial stabilizer. During the procedure, blood pressure, heart rate and oxygen saturation, as well as the dynamics of the CT segment on the ECG, were continuously measured. Results. During the experiment, there was no statistically significant change in blood pressure, heart rate, saturation and ECG. All experimental animals survived. No complications were noted. Conclusion. It can be noted that the myocardial stabilizer developed by heart team of Republican Research Center of Emergency Medicine provides excellent stabilization of the target segments of the coronary artery and facilitates vascular anastomosis on the beating heart.
Keywords: Off-pump coronary artery grafting, Stabilizer, Experimental model
Cite this paper: Abdurakhmanov A. A., Efficacy and Safety of New Myocardial Stabilizer for Off-Pump Coronary Artery Bypass Grafting, American Journal of Medicine and Medical Sciences, Vol. 11 No. 5, 2021, pp. 362-366. doi: 10.5923/j.ajmms.20211105.02.
1. Introduction
- In 1910, Carrel [1] performed the first coronary artery bypass surgery using a preserved carotid artery (CA) on a beating heart in a dog. The world's first coronary artery bypass grafting in humans was performed by Kolesov in Leningrad in 1967 [2]. In April 1968, Rene Favaloro published the first series of coronary artery bypass grafting surgeries [3]. In the 50 years since this landmark publication, coronary artery bypass grafting (CABG) has been enthusiastically developed by cardiac surgeons around the world. This was followed by the widespread use of standard coronary artery bypass grafting (CABG), which became possible due to the development of cardiopulmonary bypass techniques with various approaches with or without cardioplegic protection. Currently, most coronary artery bypass grafting (CABG) procedures are performed using cardiopulmonary bypass (CPB). The use of CPB for CABG operations allows performing anastomosis of the coronary artery in a stable bloodless surgical field with myocardial protection, providing excellent long-term results [4]. However, cardiopulmonary bypass is associated with several side effects such as bleeding, neurological complications, tissue edema, myocardial damage, and potential problems with weaning the patient from the heart-lung machine [5,6,7]. Many of these complications can be attributed to mechanical and immunological changes in blood components [8,9]. In addition, cross-clamping of the aorta during the procedure may increase the risk of myocardial, aortic, and neurological complications [10]. More recently, the development of serious persistent cognitive defects in patients undergoing cardiopulmonary bypass was noted, regardless of its duration [11].The development of the off-pump coronary artery bypass grafting technique became possible with the development of technical aspects proposed by Lima et al. In the 1990s, the technique using a series of pericardial retraction sutures allowed access to the arteries of the lateral surface of the heart [12]. Later, methods for improved visualization of the lateral wall were described using a traction suture in the oblique sinus of the pericardium [13]. Further improvement in the technique of beating heart surgery is associated with Grundeman and Borst [14-15], who improved the OPCAB operation by applying vacuum fixation technologies to expose and stabilize coronary vessels. They studied spatial motion and the biological effects of stabilized absorption in an experimental laboratory and proved the superior stabilization of this method.By 1999, according to the Society of Thoracic Surgeons (STS), beating heart bypass grafts accounted for about 10% of the total volume of CABG operations performed in the United States, and by 2007 - already about 20% [16]. And now this method has both its supporters and skeptics. Until now, OPCAB does not exceed 20-25% in the USA, 19% in the UK, 5% in Canada, reaching 60-75% in the practice of individual surgeons [17-19]. The ability to stabilize or immobilize the surgical site can significantly improve surgical accuracy and reduce the time required to complete a specific procedure [20]. Methods and device for performing CABG on a beating heart are first described in the publications of Benetti et al. [23]. In some cases, devices that provide mechanical stabilization of the myocardium by compression encounter difficulties in providing mechanical pressure on the myocardial surface [21-22]. Similarly, devices that use a vacuum (vacuum stabilizers) have great difficulty in creating and maintaining effective "suction" to the moving surface of the heart [25]. Even when the beating heart has been effectively stabilized, the target coronary artery may be covered by layers of fat or other tissue, making it very difficult for the surgeon to visualize it. Moreover, the stabilizing devices can lead to the deformation of the tissue surrounding the coronary artery, or the coronary artery itself, so that the arteriotomy remains in an unfavorable position for anastomosis.In the connection with above mentioned, it is important to have methods and devices for stabilizing the beating heart that are capable of maintaining atraumatic interaction with the surface of the beating heart over a wider range of conditions and orientations. Therefore, the development of simple and safe surgical methods that provide a stable and bloodless coronary anastomotic field is important for the successful CABG with a beating heart.The aim of the study was to evaluate the newly developed myocardial stabilizer in an experimental model in dogs.
2. Material and Methods
- This experimental study was performed in Experimental Department of Republican Research Center of Emergency Medicine (Tashkent. Uzbekistan), under the permission of the Republican Committee of Ethics during January 2021. Four mongrel dogs (12 to 20 kg) were included in this pilot study. Anesthetic management was provided with ketamine (20 mg / kg). The animals were artificially ventilated with 100% oxygen at a rate of 0.5 l / s. Neuromuscular blockers were not used in this study. Then the animals were prepared for hemodynamic monitoring. Heart rate was measured using a continuous electrocardiographic monitor (model 90903A; SpaceLabs Inc, Redmond, Washington). Arterial oxygen saturation was measured with a sensor (Pulse Oxymeter; Criticare Systems Inc, Redmond, WA) attached to the tongue (Figure 1).
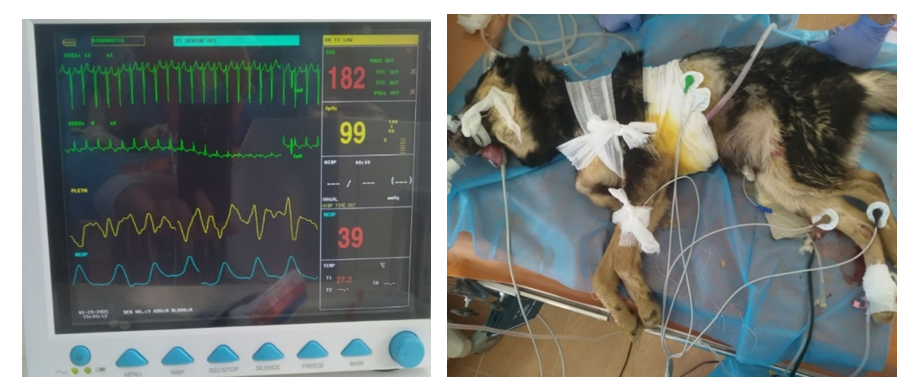 | Figure 1. Intraoperative photos describing the experimental technique and monitoring of experimental animals |
3. Results
- All animals survived the procedure and maintained stable hemodynamic parameters both during and after manipulation (Table 1).
|
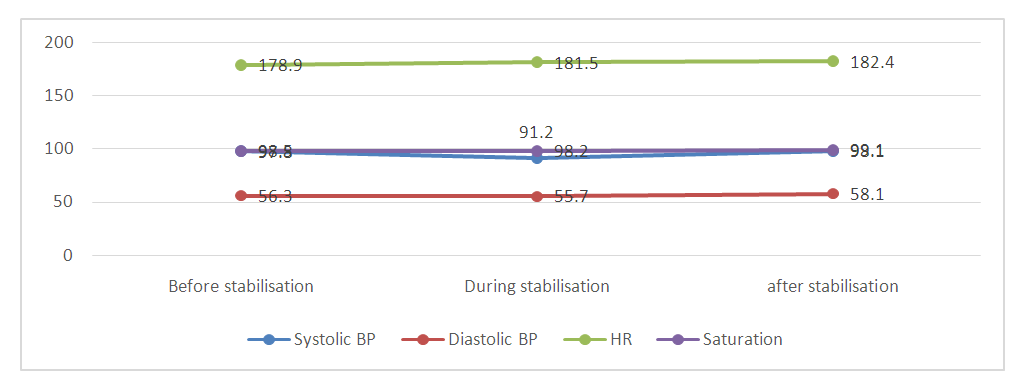 | Figure 2. Changes in key hemodynamic parameters before, during and after coronary artery bypass grafting using a myocardial stabilizer (BP - blood pressure; HR - heart rate) |
4. Discussion
- Recent studies confirm that OPCAB should be considered technically more challenging than conventional CABG. To ensure optimal conditions for surgical intervention, three fundamental rules must be followed: the surgical field must be well open and accessible, stable and bloodless. During OPCAB, cardiac surgeons voluntarily violate these rules. However, to date, none of the available mechanical stabilization devices has been able to achieve a stable bloodless field comparable to the surgical field achieved with cardioplegic cardiac arrest and cardiopulmonary bypass. Moreover, a number of surgeons express doubts about the quality of coronary anastomoses in interventions on a beating heart. There is still no completely clear evidence. In 2001, Puskas et al. [28] reported an impressive 98% OPCAB patency rate at hospital discharge, while more recently Khan et al. [29] reported the results of a prospective randomized study with a graft patency rate at three months of 98% for patients operated on -pump, compared with 88% for patients operated off-pump (P = 0.002). The learning curve appears to play an important role in OPCAB, and many series have been published showing higher patency rates and lower incidence of unsatisfactory anastomoses as the surgeon grows in experience [30-31].The movement of the heart occurs in three dimensions of space and can be described as a smoothly varying combination of sinusoidal waves, we found only a few publications in which the movement of the surface of the heart is quantified [32]. In a pig model, Borst et al. [33] calculated the two-dimensional area (x and y axes) covered by the control point on the epicardium during free heartbeat and after the installation of a mechanical vacuum stabilizer, showing a significant reduction in movement (from 73 ± 43 mm2 to 1.3 ± 0.5 mm2) at stabilization of the heart. Koransky et al., [34] analyzed the three-dimensional movement of the coronary artery LAD in pigs using sonomicrometry techniques. Movement and speed were analyzed alternately in the x, y and z planes using triangulation theory before and after placement of the vacuum stabilizer. Stabilization led to a significant decrease in travel (11.36 ± 1.74 versus 5.99 ± 1.30 mm; p <0.05), maximum Cartesian speed (141.80 ± 29.73 versus 86.55 ± 29.45 mm / s; p <0.05) and the average Cartesian LAD velocity (44.30 ± 7.02 versus 21.46 ± 4.54 mm / s; p <0.05). In their most recent study, Cattin et al., [35] showed significant reductions in maximum excursion, excursion speed, and trajectory deviation after LAD stabilization with an Octopus device in three pigs. These authors used an 8-bit high-speed black and white video camera (50 frames/s) in combination with a laser sensor (60 μm resolution) to capture the movement of the heart wall around the LAD in all three dimensions. In their experiments, the residual excursion of the coronary artery after stabilization was about 300–330 µm in the x and y plane and about 2–2.6 mm in the z plane.The myocardial stabilizer developed by our team allows performing coronary artery bypass grafting on a beating heart. No undesirable effects on any of the measured hemodynamic parameters were observed when using this stabilizer. Despite the small diameter of the coronary arteries in dogs and a high heart rate, coronary artery bypass grafting was successful and was not accompanied by complications and mortality in the experimental part of the work.
5. Conclusions
- It can be noted that the myocardial stabilizer developed by heart team of Republican Research Center of Emergency Medicine provides excellent stabilization of the target segments of the coronary artery and facilitates vascular anastomosis on the beating heart.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML