-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(4): 322-327
doi:10.5923/j.ajmms.20211104.14
Received: Mar. 1, 2021; Accepted: Apr. 2, 2021; Published: Apr. 26, 2021

The Problem of Food Allergies and Cross-Allergic Reactions to Fungi and Latex
Abdullaeva Dilafruz1, Iskandarova Gulnoza2, Abdullaev Mansur2, Khakberdiev Khusan1, Ashurov Jamshidbek Bakhtiyor Ogli1
1Department of Hygiene of Children, Adolescents and Food Hygiene, Tashkent Medical Academy, Tashkent, Uzbekistan
2Department of Epidemiology, Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
Correspondence to: Abdullaeva Dilafruz, Department of Hygiene of Children, Adolescents and Food Hygiene, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Allergy is a global public health problem nowadays and needs to be addressed both at the level of each country and globally. Clinical manifestations of food allergy covers a wide range of diseases of the skin, gastrointestinal and respiratory tract. Oral allergic syndrome (OАS) is one of the manifestations of food allergy, which is associated with sensitization to pollen. Homologous proteins found in plant foods can cause allergic reactions. Today, diet therapy is the main treatment for food allergy. The basic principle of the diet for food allergy is the elimination of allergens and allergen components that are distinctive to each patient and considering with his climatic conditions and the eating peculiarity.
Keywords: Food allergy, Food allergens, Syndrome of cross-reactivity, Immunoglobulin E, Fungi, Latex
Cite this paper: Abdullaeva Dilafruz, Iskandarova Gulnoza, Abdullaev Mansur, Khakberdiev Khusan, Ashurov Jamshidbek Bakhtiyor Ogli, The Problem of Food Allergies and Cross-Allergic Reactions to Fungi and Latex, American Journal of Medicine and Medical Sciences, Vol. 11 No. 4, 2021, pp. 322-327. doi: 10.5923/j.ajmms.20211104.14.
Article Outline
1. Introduction
- Food allergy (FA) is a widespread disease with prevalence ranging from 0.1% to 50% among population. Clinical manifestations of food allergy covers a wide range of diseases of the skin, gastrointestinal and respiratory tract. Oral allergic syndrome (OАS) is one of the manifestations of food allergy, which is associated with sensitization to pollen. OAS is characterized by homology of thermo labile proteins of plant pollens, fresh fruits, and vegetables.An understanding the features of the clinical presentation can help with diagnosis. Diagnostic testing is highly sensitive and often identifies clinically irrelevant sensitization. Testing therefore must be selected and interpreted in the context of the patient’s clinical history. Prognosis varies depending on the food allergen, and food allergy is a risk factor for other atopic diseases [3].Pollen-food allergy syndrome (OAS) is a common disorder in which oral symptoms, pruritus, itching of the throat and occasionally mild oral edema occur immediately upon the ingestion of certain foods, most commonly raw fruits and vegetables, but also certain nuts, e.g. hazelnuts and peanuts can trigger these symptoms. These complaints are due to specific IgE antibodies directed to aeroallergens that cross-react with certain food proteins. Skin tests are often performed with fresh fruits and vegetables to confirm the diagnosis, and the component diagnostic protein tests for hazelnut (Cor a 9 and 14) and peanut (Ara h 8) proteins will reveal IgE to the cross-reactive protein of birch pollen Bet v 1 [1]. OAS is an allergic reaction in the oral cavity after the consumption of food, such as fruits, nuts, and vegetables, which occurs in adults who suffer from allergic rhinitis. Here we investigate the prevalence and triggers of birch pollen-related food allergy. It has been described under various names including «pollen-food allergy syndrome», «pollen-food syndrome» and «pollen-associated food allergy syndrome» [8,9,11]. OAS in adults probably represents the most common allergic reaction caused by food; and more than 60% of all FA are actually cross-reactions of food and inhaled allergens. Unlike other food allergies, OAS is a reaction limited to the oral mucosa, lips, tongue, and throat. OAS belongs to the allergy type I group, that is, allergic reactions mediated by immunoglobulin E (IgE). In susceptible patients, the immune system produces IgE antibodies against the proteins of pollen which causes hay allergy. Pollen allergies are caused by repeated exposure to the pollen of some plants, which are usually pollinated via air and have such pollen quantities that inhalation of the pollen easily reaches the surface of the pulmonary alveoli. The proteins which are structurally similar to pollen are also found in food [8,11,17].The OAS, more common in adults and adolescents manifests the symptoms mainly affecting the oropharynx, and depends on a polysensitization to inhalant and food allergens. Raw apple, peanuts, almonds, hazelnuts, and other fruits of the Rosaceae family are commonly causes the allergic reaction in patients with an allergy to a birch, while banana, kiwi, and melon are the products causing the allergic reaction in allergic patients to ambrosia, melon and tomato are responsible for symptoms in grass allergy patients. In this phenotype, the IgE against pollen cross-reacts with homologous proteins in plant foods [10].Clinical symptoms of FA cover a wide range of diseases of the skin, gastrointestinal and respiratory tract, one of the most common among them is OAS associated with sensitization to pollen. OAS is characterized by homology of thermolabile proteins of plant pollen, fresh fruits, and vegetables. Thus, 23-76% of patients with allergic rhinitis in different countries have a history of allergy symptoms to at least one food, and more than half of patients with OAS suffer from intolerance to more than two types of plant foods. In European countries, 70% of patients with sensitization to birch pollen have OAS associated with the fruits from the Rosaceae family, such as apples, peaches and pears. In this regard, the high prevalence of cross-reactivity to food of plant origin in patients with OAS requires a need to develop innovative diagnostic methods and an algorithm for providing medical and preventive care.Today allergy is a global public health problem and needs to be addressed both at the level of individual countries and the world community. It should be noted that the diagnostic value of immunological tests in vitro diagnostics is quite high and amounts to 87-90%, the information about the content of skin tests with food allergens is only 49% [16]. According to foreign authors, all allergens, including household pollen, fungal and food allergens are divided into 130 protein families, depending on the functional properties and the similarity of the amino acid sequence. Currently, more than 130 allergenic molecules are available for in vitro diagnostics of specific immunoglobulin E (IgE) [14,19].The aim of the study was to study important allergens that are distinctive for the country, identify hidden allergens and interpret the results for the diagnosis of type I allergic reactions. It is known that the molecules of allergens differ depending on the biological function as well as the structure of the protein families. Some molecules have common epitopes (antigen binding sites), and IgE antibodies can bind to allergen molecules that have identical structures. Their origin can be different, the identification of allergens, with cross-reactivity is significantly important and will give clinicians information about the sensitization of the patient's body to different allergens.It is possible to predict the tendency to develop a systemic or local reaction and the persistence of clinical symptoms with the information about which molecules of allergens the patient is sensitized to. The high prevalence of allergic diseases in our republic requires the development of innovative diagnostic methods, which have a particular social and medical significance.
2. Materials and Methods
- In the clinics of the republic, after a thorough analysis of the anamnesis, clinical symptoms were revealed in patients aged 14-70 years (n = 334) with allergic diseases (food allergy, allergic rhinitis, bronchial asthma, atopic dermatitis), and also the level of specific IgE antibodies to food, vegetable, professional (latex), household and fungal and allergens typical for the region has been determined.Execution technique: The test is based on the principle of immunoblotting. Various specific allergens corresponding to the composition of the panel are applied to the surface of the nitrocellulose membranes in separate stripes. The test device is a strip of nitrocellulose membrane 4 mm wide and 64 mm long, coated with allergen material in the form of 20 test strips and 5 standard strips, the strip is enclosed in a plastic case 12 mm wide, 93 mm long and 3.4 mm thick. Each panel is easy to use and varies in color.If IgE antibodies specific to these allergens are present in a patient’s sample, they react with the corresponding band. At the second stage of incubation, biotin-conjugated antibodies to human IgE bind to the formed allergen-IgE complexes. During the third stage of incubation, peroxidase-conjugated streptavidin (conjugate) is attached to biotin. The enzyme converts the colorless substrate tetramethylbenzidine (TMB) into a lilac end product. Each incubation step is followed by a washing step, during which all unbound components are removed. The intensity of the lilac color is directly proportional to the amount of allergen-specific antibodies in the serum. Evaluation of the result is carried out on a conventional color flatbed 3 – d scanner, in conjunction with the RIDA qLine®Soft software. The intensity of the staining of the allergen bands can be quantified based on the standard curve located on each strip and expressed in the corresponding units IU / ml or RAST classes (radioallergosorbent test). The measured values are used to automatically calculate the concentration of s IgE in each band in the SI system (IU / ml) and assign the PAST allergenic reactivity classes from 0 to 6. The assessment is carried out according to the standard curve contained on each strip, while the intensity of each individual allergen on the strip is compared precisely with this standard curve.To determine s IgE to various allergens, fasting blood was taken in the morning from the cubital vein into plastic tubes without a clotting catalyst in a volume of 5 ml. The blood was left to clot at room temperature for 30 minutes, then centrifuged at 2000 rpm for 10 minutes to obtain serum. For each determination, 400 μl of serum was used. The concentration of allergens was determined using a special program on R-Biopharm equipment.Ethical considerationsThe research has been allowed by Republican ethical committee for ethical considerations. Every patient has been asked to give permission before being researched.
3. Research Results
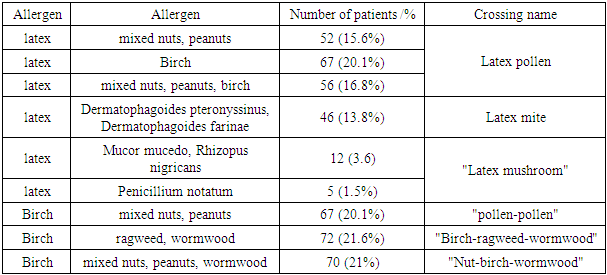
- The results of a study using allergenic panels, Rida qLine Allergy (Germany) showed that 67 (20.1%) patients had latex sensitization, 33 (9.8%) patients had allergic reactions accompanied by «latex – pollen», «latex – fruit» syndromes. At the same time, the following tendency was observed: the likelihood of the manifestation of the «latex-fruit» syndrome was lower in adolescence than in adults, which may be associated with a different frequency of contact with latex. In 46 (13.8%) patients, specifiс IgE to latex and to mites of the genus Dermatophagoides pteronyssinus and / or Dermatophagoides farinae – «latex-mite» syndrome was detected; in 12 (3.6%) - for latex and fungi of the genus Mucor mucedo, Rhizopus nigricans, in 5 (1.5%) - for latex and fungi of the genus Penicillium notatum, which proves the presence of «latex-mushroom» syndrome and cross-allergic reactions between latex and household mites of the genus Dermatophagoides pteronyssinus and / or Dermatophagoides farinae and fungi.We identified cross-reactions with latex in 15.6% of patients to nuts (hazelnuts, almonds, Brazil nuts, coconut, walnuts) and peanuts. Specifiс IgE antibodies to latex and birch were present in 20.1% of patients.As the test results showed, the patients showed cross-reactions between allergens: strawberries and peaches (29.4%), between Rosaceae and honey (20.6%), between peanuts and coffee (11.8%). Common allergens include fruits from the Rosaceae family: peaches, apples, pears, apricots, strawberries and other stone fruit trees. Allergy to apples in many patients is combined with allergy to birch pollen due to the identity of their allergenic proteins, symptoms of damage to the oral cavity and pharynx are mainly observed, sometimes it can be accompanied by allergic rhinitis, bronchial asthma and gastrointestinal disorders.Mushroom allergens include about 350 species of fungi that have been found to have allergic properties. Fungi are eukaryotic, single- or multicellular organisms that exist as saprophytes or parasites of plants and animals. The structure of fungal spores differs from pollen spores, since the inhalation particles of fungi consist of living cells capable of growing and secreting allergens in vivo [4].Families of fungi that are widespread in our climate which give cross-reactions and cause sensitization in patients with AD are now known. Of all the data obtained in the republic for positive results on RAST within the range of 1-6, the largest percentage of fungi was found for Mould fungi: Mucor mucedo, Rhizopus nigricans (14.1%). And also IgE indices were detected for fungi: Cladosporum herbarum (4.8%), Alternaria alternata (5.7%), Aspergilus nigrus (2.7%), Penicillinum notatum (7.5%), Candida albicans (3%). Sensitization to molds (Mucor mucedo, Rhizopus nigricans) was revealed in 14.1% of patients, in 5.7% - to Alternaria Alternata and in 7.5% of Penicillinum notatum, which is characterized as a «latex-mushroom» syndrome. Sensitization to house dust mites Dermatophagoides farinaе (d2) was noted in 18.6% of patients and to Dermatophagoides pteronyssinus (d1) in 29.3%. The patients had clinical signs in the form of allergic rhinitis, conjunctivitis, contact dermatitis, urticaria, bronchial asthma (Table 1).
|
4. Discussion
- According to foreign authors, individuals with allergies to Rosaceae, especially in the case of sensitization to PR-10 proteins (apple - Mal d 1, peach - Pru p 1) or profilins (peach - Pru p 4), more often have a local reaction, because these proteins are destroyed by high temperatures and digestive enzymes. Sensitization to lipid transfer (LTP) proteins (peach - Pru p 3), characterized by clinical manifestations of a different nature (asymptomatic course, anaphylaxis), as a rule, is a marker of anaphylaxis, which is provoked by other factors (physical activity, medication, wheat, etc.) [13].In patients with pollen allergies, cross-reactivity between airborne allergens and food can cause FA. Symptoms can range from oral allergy syndrome to severe anaphylaxis. Clinical manifestations associated with IgE sensitization to cross-reactive components of aeroallergens and food allergens have been characterized for many plant sources (pollen-food syndromes and associations, such as birch-apple, cypress-peach and celery-mugwort-spice syndromes, and mugwort-peach, mugwort-chamomile, mugwort-mustard, ragweed-melon-banana, goosefoot-melon associations), fungal origin (Alternaria-spinach syndrome), and invertebrate, mammalian or avian origin (mite-shrimp, cat-pork, and bird-egg syndromes) [7].Global climate changes have affected the amount of pollen, its allergenicity, and the duration of the dusting season. The use of new technologies in the food industry has radically changed the concept of the composition of a particular product. Animal products may contain plant allergens (for example, sausages may contain soy proteins, spices, etc.) [12].Prior to OAS, sensitization to pollen containing proteins homologous to certain fruits and vegetables often develops. In such a case, a pollen sensitive patient may react to a food allergen without first contacting it. For example, sensitization to birch pollen can be combined with OАS after eating Rosaceae family fruits, especially apples, peaches and cherries. In practice, cross-reactions between latex and banana, avocado, peach, kiwi, apricot, grapefruit, pineapple cases are described.There is cross-reactivity among the carrots, walnuts, peanuts, celery, apples, cherries, pears, buckwheat and pollen of birch; watermelon, banana, sunflower seeds, honey and chamomile with ragweed pollen. Watermelon, oranges, cherries, potatoes provide cross reaction with pollen of grasses. This is called «pollen-fruit» syndrome. Celery can cause both oropharyngeal symptoms and system reactions like urticaria, asthma and anaphylactic shock. The latex allergy problem is an example of a «new allergy» that suddenly emerges with enormous consequences for the health of patients and the economy. More than 12 million tons of natural latex are produced annually from rubber, but a limited number of latex-derived products have been approved and regulated by government agencies such as the FDA. The problem is that most finished products are not labeled for latex. Latex-fruit syndrome is a type of cross-reaction, for example sensitization to Hev b 8 (profilin), has no clinical significance. Sensitization to other latex proteins can cause clinical manifestation, the relationship between allergens and the severity of clinical manifestations has no correlation. The cross-reactive allergen that responsible for the development of latex-fruit syndrome has not yet been determined, although it is believed that these could be latex proteins Hev b 5, 6 and 11. Recent studies have contributed to the characterization of 15 latex allergens and to analyze specific IgE antibodies against components of latex allergens. Significant progress has been made in understanding and preventing latex allergy, although the disease may still be a cause of concern worldwide [15,20,21,22].Proteins of natural rubber latex and certain fruits, vegetables (banana, kiwi, tomatoes, potatoes, carrot and watermelon) contain homologous proteins, which can cause «latex-fruit» syndrome in sensitized patients. The tactics of prescribing an elimination diet, which takes into account the exclusion of causally significant allergens from the patient's diet, primarily depends on the timely diagnosis of causal allergens, the severity of clinical symptoms, and the patient's age. Establishing the «culprit» - allergen and knowing its ability to cross-reactivity, resistance to heat treatment, as well as the correct determination of the appropriateness and duration of elimination of the allergen is an important task of the nutritionist-allergist.In our country, the diagnosis of allergic diseases is difficult due to the lack of unified methodological approaches and standardized diagnostic methods to determine the mechanisms of allergy development. It should be noted that pseudo-allergic reactions are often clinically identical with allergic ones, but there are differences in their mechanisms. So, during pseudo-allergic reactions, specific antibodies are not formed, the reaction depends on the dose of the consumed food product and, unlike allergic reactions, is triggered at the first contact.The same problem can arise with two well-known types of FA; wheat-dependant exercise induced anaphylaxis and allergy to non-specific Lipid Transfer Protein allergens, both of which might only manifest when linked to a cofactor such as exercise. Many of these risk factors for food anaphylaxis have a common link; the public's engagement with popular concepts of health and fitness. This includes the development of a food and exercise culture involving the promotion and marketing of foods for their health-giving properties i.e., meat substitutes, wheat substitutes, supplements and alternative, or “natural” remedies for common ailments. Some of these foods have been reported as the cause of severe allergic reactions, but because they are often viewed as benign unlikely causes of severe allergic reactions, could be considered to be hidden allergens [18]. Allergenic proteins can contain linear or conformational epitopes or can be heat stable or heat labile. Food allergens can be modified by food processing or are affected by specific methods of cooking, which can denature the protein or, conversely, render a protein more allergic through various known chemical pathways [5].Among the allergenic proteins of animal origin, the most clinically significant are caseins and lipocalins: the main milk allergens, a family of protease inhibitors, and calcium-binding proteins: parvalbumin, the dominant fish allergen [2].As you know, milk and its processed products are widely used in the confectionery industry. Thermally stable milk protein casein enhances moisture retention in sweets and candies, in melted dairy products it improves crust color and strength, milk proteins serve as a whipped marshmallow base. Casein and caseinates are often used as salad dressings, fillers and spices in sausages, soups and stews, sauces, and ice cream [4]. Some food products (cow’s milk, peanut, egg) contain several epitopes, ensuring their allergenicity. Increased sensitization to chicken eggs, especially chicken protein, is a widespread problem that affects 1-2% of children worldwide. Mainly 4 egg allergens: ovomucoid, ovalbumin, ovotransferrin and lysozyme cause hypersensitivity in patients [6]. Meat is a histamine liberator, rarely causes allergies, consumption in large quantities can lead to the development of pseudo-allergic reactions, possibly due to the effect on mast cells by a non-specific mechanism. The antigenic composition of the meat of different animals differs, therefore, in patients with beef allergy, chicken, pork and lamb meat may not cause an allergy. Lamb is considered a mild food allergen, but shares common allergens with beef and sheep wool. The allergenic potential of meat is reduced by heating and by exposure to pepsin. This variant of sensitization in the determination of simultaneously specific IgE (sIgE) antibodies to egg and chicken meat in medicine is called the «bird-egg» syndrome.Patients who are allergic to chicken meat may react to meat from other birds such as turkey. Albumin of different animals has a high degree of structural similarity and in the presence of hypersensitivity to albumin of one species of animal, patients may react to the epithelium and meat of other animals [4]. Haptens, which can combine with other dietary proteins, can also become complete allergens. When the immune barrier of the gastrointestinal tract is disturbed, a huge amount of antigens can enter the body. With the normal functioning of the gastrointestinal tract and the hepatobiliary system, sensitization to food supplied by the enteral route does not develop [6]. When determining specific antibodies of the IgE class, the nature of allergic food intolerance is confirmed, which makes it possible to compile a list of products that are contraindicated for patients. Eating an appropriate diet is considered the primary way to truly prevent food allergies. In our republic, the main food allergens include cow's milk, eggs, cereals, gluten, peanuts, nuts, sesame seeds, buckwheat, celery. However, individual selection of the most likely food allergens for testing should be based on the patients' specific diet.Based on the above, the development of dietary rations, considering the climatic features of patients with food allergies, the component composition and allergenic properties of foods, with the help of modern methods of diagnosis becomes actual and perspective. After determining the cause-significant allergens, it is recommended that they will be completely eliminated from patient's diet. It is necessary to avoid skin, inhalation ways of getting these allergens into the body of sensitized patients. The diet should be strictly individual and it is necessary to make a diet, being guided by the anamnesis, clinical symptoms, age of the patient taking into account cross reactions between allergens. Thus, we can conclude that the creation of dietary rations taking into account the climatic properties of the region, the individual peculiarities of patients for food allergies, the component composition and allergenic properties of food products using modern diagnostic methods is becoming relevant and promising. Attention should be drawn to the fact that the diet should be strictly individual and when compiling it, it is necessary to be guided by the history, clinical symptoms, age of the patient, taking into account cross-allergic reactions between different groups of allergens. For all patients, it cannot be a single «standard» elimination diet.
5. Conclusions
- Based on the above, the development of diet, considering the climatic features of patients with food allergies, the component composition and allergenic properties of food, with the help of modern methods of diagnosis became an actual and perspective. As a result of many years of research, we have recommended a list of «guilty» food products with high allergenic activity in our republic, such as cow's milk, eggs, cereals, gluten, peanuts, nuts, sesame seeds, buckwheat and celery. Due to the fact that these food products have major allergenic components, the problem of managing latent allergens and their labeling is an urgent task of medicine.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML