-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(4): 316-321
doi:10.5923/j.ajmms.20211104.13
Received: Feb. 15, 2021; Accepted: Apr. 12, 2021; Published: Apr. 26, 2021

The State of Renal Functional Reserve During the Predialysis Stages of Chronic Kidney Disease
Barnoev Khabib1, Sabirov Maksud2, Munavvarov Burhonjon2
1Department of Nephrology, Multidiscipline Clinic of Tashkent Medical Academy, Tashkent, Uzbekistan
2Department of Therapeutic Subjects №2, Tashkent State Dental Institute, City Tashkent, Uzbekistann
Correspondence to: Barnoev Khabib, Department of Nephrology, Multidiscipline Clinic of Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents the results of a study to assess the functional reserve of the kidneys in a comparative study of antiaggregant therapy with pentoxifylline and alltrombosepin in 60 patients with a relatively early stage of chronic kidney disease. Studies have shown that long-term administration of alltrombosepin to patients has resulted in better maintenance of renal functional reserves. Therefore, pentoxifylline, which is widely used as an antiaggregant drug in chronic kidney disease can be easily matched by alltrombosepin, which is produced in our country from local raw materials - fact that was also confirmed during our research.
Keywords: Chronic kidney disease, Renal functional reserve, Glomerular filtration rate, Antiaggregant, Alltrombosepin, Pentoxifylline
Cite this paper: Barnoev Khabib, Sabirov Maksud, Munavvarov Burhonjon, The State of Renal Functional Reserve During the Predialysis Stages of Chronic Kidney Disease, American Journal of Medicine and Medical Sciences, Vol. 11 No. 4, 2021, pp. 316-321. doi: 10.5923/j.ajmms.20211104.13.
Article Outline
1. Introduction
- Despite the beginning of the new century, the problems of the transition and treatment of chronic kidney disease have not been adequately addressed [3,8,20]. The disease is characterized by severe and irreversible damage to many pathogenic joints. One of the most important of these is the disruption and disproportion of hemostasis. According to the National Kidney Foundation, all patients with chronic kidney disease should be included in the high-risk group for the development of cardiovascular disease, in which traditional factors do not play a significant role [12,13,17,21]. Hemostasis is an important pathogenetic link in the development of cardiovascular diseases in chronic kidney disease [4,7,12].Ball filtration rate (BFR) evaluates the simultaneous function of kidneys, which is influenced by several factors and even when there are reliable markers, true filtration is still maintained at the norm because it does not lose up to 50% of the nephrons. Functional reserve of the kidneys is a response to physiological or pathological stimulation of the renal working capacity by increasing the rate of ball filtration [5,11,15,19].At present, various anti-aggregant drugs are used in the treatment of chronic kidney disease in the world community. Of these, pentoxifylline is widely used in nephrological patients, has a high response rate and is included in the standard of treatment [6,10,18]. At the same time, the drug alltrombosepin, which is derived from local raw materials and has undergone a number of laboratory and clinical trials, is used in cardiology as an antiplatelet agent. The main essence of our research is to improve the state of hemostasis and blood rheology in patients with relatively early stage of chronic kidney disease, as well as to reduce the aggregation of trace elements. Thus, in the treatment of chronic kidney disease, blood rheology-enhancing and antiplatelet agents are important. Therefore, we have conducted research to monitor and evaluate the effect of antiplatelet drugs pentoxifylline and alltrombosepin on the functional reserve of the kidneys in chronic kidney disease.
2. The Purpose of the Study
- Evaluation of the effect of antiplatelet therapy on the functional reserve of the kidneys in patients with stage II and III renal disease on the basis of comparative observations in groups with the addition of pentoxifylline and alltrombosepin.
3. Research Material and Methods
- For the study, 60 patients with chronic kidney disease, treated in the nephrology department of the Multidisciplinary Clinic of the Tashkent Medical Academy, developed as a result of nephropathy of various genesis (creatinine glomerular filtration rate (GFR) 30-89 ml/min/m2) were selected. Groups of 1 (n-30) and 2 (n-30) were selected from 60 patients who could be tested for RFR, ie those without massive tumors, severe and moderate symptomatic arterial hypertension, in short, whose body was able to withstand protein loading. Group 1 consists of CKD (chronic kidney desease) patients who have started taking pentoxifylline. The second group consisted of patients receiving alltrombosepine 200 mg/day (1 capsule of the drug in a dose of 100 mg, prescribed 1 capsule 2 times a day for 3 months).On the first day RFR of all patients was calculated by protein loading through parenteral administration of amino acid solution (Akumin-Nephro 1000 ml). RFR was calculated using the following formula [1]:RFR = (GFR2 – GFR1)/GFR1) x 100%,• GFR1 is the starting dose of GFR.• GFR2 - the amount of GFR after protein transfer.GFR, urea and creatinine in whey, and total protein and proteinuria, which are directly related to the dynamics of protein intake and are directly related to the calculation of RFR, were not excluded. Doppler examination of the renal arteries was also performed. GFR was calculated using the formula CKD-EPI (2009) modified in 2011 for the amount of creatine in whey (using an on-line calculator at http://nefrosovet.ru/). In it, the indicators of resistance (RI) and pulse (PI) index, calculated from the main gradients that determine the level of vascular resistance, were studied in the main and segmental renal arteries. Ninety days after treatment in groups 1 and 2, the same test and analysis were re-examined. The obtained results were statistically analyzed.
4. Results
- In the analysis conducted to assess the functional reserve of the kidneys in group 1 patients, the results were as follows. According to this, on the first day of treatment, the amount of creatinine before protein loading was 169.4 ± 12.41 μmol/l, and after protein loading, creatinine decreased to 164.1 ± 10.72 μmol/l. At 90 days of treatment, creatinine before protein loading was 156.6 ± 7.05 μmol/l, and after protein loading, creatinine decreased to 151.4 ± 7.22 μmol/l.Urea in the blood was 11.1 ± 0.47 mmol/l before protein loading on the first day, but decreased to 10.4 ± 0.34 mmol/l after protein loading. By 90th day of treatment, the value of urea was 9.0 ± 1.55 mmol/l before the protein load and the urea after the protein load was 8.9 ± 0.46 mmol/l.GFR was 42.1 ± 2.42 ml/min before protein loading at the beginning of treatment and increased to 44.9 ± 2.21 ml/min after protein loading. By 90th day of treatment, GFR was 44.9 ± 1.55 ml/min before protein loading and 47.8 ± 1.32 ml/min after protein loading.Thus, when patients were given a protein load, the oncotic pressure of plasma increased due to an increase in total protein in the blood. As a result, an increase in GFR was observed as a result of the addition of reserve glomerulus to the activity, and a consequent decrease in products such as urea and creatinine in the blood.Proteinuria at the beginning of treatment was 1.9 ± 0.78 before protein loading; after protein loading, it increased to 1.94 ± 0.64. By the 90th day of treatment, however, proteinuria was 0.86 ± 0.282 prior to protein loading; an increase of 0.90 ± 0.218 was observed after protein loading. This condition is due to the tension of the damaged nephrons during protein loading.Total protein was 61.8 ± 1.72 g/l before protein loading at the beginning of the study, but increased to 62.4 ± 1.33 g/l after protein loading. By the 90th day of treatment, total protein was 63.0 ± 1.43 g/l before protein loading and increased to 64.6 ± 1.35 g/l after protein loading.Indicators determining the level of renal vascular resistance, resistance and pulse indices were studied in the main and segmental renal arteries. According to it, the resistance index in the main renal artery was 0.70 ± 0.018 before the protein load at the beginning of treatment, and it decreased to 0.68 ± 0.015 after the protein load. After 90 days of treatment, this figure showed a value of 0.65 ± 0.021 before protein loading and decreased to 0.62 ± 0.016 after protein loading. The pulse index was 1.60 ± 0.23 before protein loading at the beginning of treatment and it was seen to decrease to 1.54 ± 0.32 after protein loading. After 90 days of treatment, this value was 1.49 ± 0.24 before the protein load and decreased to 1.45 ± 0.21 after the protein load.In the segmental renal arteries, the resistance index was 0.69 ± 0.018 before protein loading at the beginning of treatment and decreased to 0.68 ± 0.020 after protein loading. After 90 days of treatment, this figure showed a value of 0.64 ± 0.014 before protein loading and decreased to 0.62 ± 0.016 after protein loading. The pulse index was 1.21 ± 0.019 before the protein load at the beginning of treatment and was seen to decrease to 1.20 ± 0.021 after the protein load. After 90 days of treatment, this value was 1.16 ± 0.012 before protein loading and decreased to 1.14 ± 0.017 afterwards. Resistance and pulse indices are related to blood viscosity and vascular resistance. When patients were given a protein load, the oncotic pressure of plasma increased due to an increase in albumin and total protein in the blood. As a result, due to the relative improvement of hemodynamics resulting from the increase in total circulating blood volume caused by the return of tissue plasma into the blood vessel, a positive shift in these indicators was observed. However, this shift is based on the fact that the reliable improvement in patients receiving pentoxifylline for three months is due to a decrease in the aggregation of trace elements and the vasodilator properties of the drug.In group 1 patients receiving pentoxifylline as an antiplatelet therapy, RFR based on blood creatinine clearance was 6.7% before treatment and increased by 7.1% after 90 days of treatment.We now present the results in the analyzes performed to assess the functional reserve of the kidneys in group 2 patients receiving the drug alltrombosepin as an antiaggregant treatment. According to it, on the first day of treatment, the amount of creatinine before protein loading was 166.5 ± 10.87 μmol, while after protein loading creatinine decreased to 160.6 ± 11.43 μmol. By the 90th day of treatment, the creatinine level before protein loading was 158.9 ± 7.09 μmol, while after protein loading, creatinine decreased to 153.4 ± 6.31 μmol.Urea in the blood was 11.3 ± 0.45 mmol/l before protein loading on the first day, but decreased to 10.4 ± 0.34 mmol/l after protein loading. By the 90th day of treatment, the urea value was 9.0 ± 1.52 mmol/l before protein loading and after loading went down to 8.8 ± 0.52 mmol/l.GFR was 42.1 ± 2.16 ml/min before protein loading at the beginning of treatment and increased to 44.8 ± 2.32 ml/min after protein loading. By the 90th day of treatment, GFR was 44.8 ± 1.28 ml/min before protein loading and 47.9 ± 2.08 ml/min after protein loading.Thus, the oncotic pressure of plasma increased due to an increase in total protein when patients were given a protein load. As a result, kidney function indicators, such as GFR, urea, and creatinine, have changed for the better as a result of the addition of reserve glomerulus to the activity.Proteinuria was 2.3 ± 0.63 before protein loading at the beginning of treatment; after protein loading, it increased to 2.32 ± 0.64. By the 90th day of treatment, however, proteinuria was 1.72 ± 0.72 before protein loading; increased by 1.73 ± 0.311 after protein loading. This condition is due to the tension of the damaged nephrons during protein loading.Total protein was 62.3 ± 1.68 g/l before protein loading at the beginning of the study, but increased to 63.2 ± 1.24 g/l after protein loading. By the 90th day of treatment, total protein was 64.1 ± 1.39 g/l before protein loading and increased to 65.2 ± 1.33 g/l after protein loading.According to the results of renal vascular dopplerography, the resistance index in the main renal artery was 0.69 ± 0.022 before protein loading at the beginning of treatment and decreased to 0.68 ± 0.012 after protein loading. After 90 days of treatment, this figure showed a value of 0.64 ± 0.019 before protein loading and decreased to 0.62 ± 0.016 after protein loading. The pulse index was 1.59 ± 0.28 before the protein load at the beginning of treatment and was seen to decrease to 1.52 ± 0.22 after the protein load. After 90 days of treatment, this value was 1.46 ± 0.16 before protein loading and decreased to 1.43 ± 0.19 after protein loading.In the segmental renal arteries, the resistance index was 0.69 ± 0.022 before protein loading at the beginning of treatment and decreased to 0.68 ± 0.012 after protein loading. After 90 days of treatment, this figure showed a value of 0.64 ± 0.019 before protein loading and decreased to 0.62 ± 0.016 after protein loading. The pulse index was 1.20 ± 0.024 before protein loading at the beginning of treatment and it was seen to decrease to 1.19 ± 0.018 after protein loading. After 90 days of treatment, this value was 1.15 ± 0.016 before protein loading and decreased to 1.14 ± 0.017 after protein loading. It is known that resistance and pulse index are related to blood viscosity and vascular resistance. When patients were given a protein load, the oncotic pressure of plasma increased due to an increase in total protein in the blood. As a result, due to the relative improvement of hemodynamics resulting from the increase in total circulating blood volume caused by the return of tissue plasma into the blood vessel, a positive shift in these indicators was observed. However, we interpret this shift as a significant improvement in patients receiving alltrombosepin for three months due to a steady decrease in the aggregation of shaped elements over months.In group 2 patients receiving alltrombosepin as an antiplatelet therapy, RFR based on blood creatinine clearance was 6.6% before treatment and increased up to 7.2% after 90 days of treatment.Looking at the results, we observed that the changes were close to each other when we followed the dynamics of the indicators assessing the functional status of the kidneys in both research groups. Although no significant positive shift was observed in both groups when calculating RFR according to creatinine clearance, it is also important that RFR persists for some time against the background of treatment with antiplatelet agents [9,21]. The dynamics of RFR in the background of treatment with antiplatelets is illustrated in Figure 1 on the basis of horizontal and vertical diagrams:
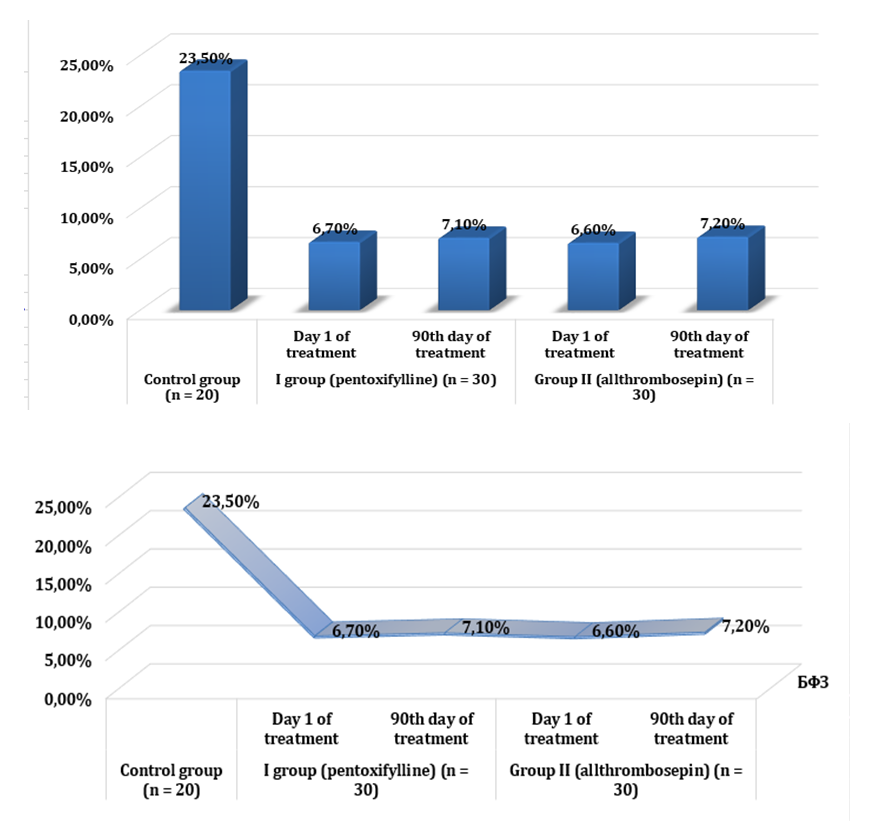 | Figure 1. Dynamics of the functional reserve of the kidneys against the background of treatment with antiplatelet agents |
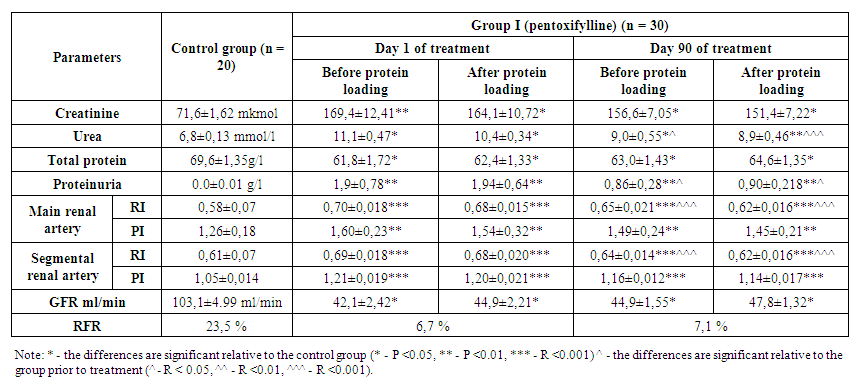 | Table 1. Results of laboratory tests of group I patients |
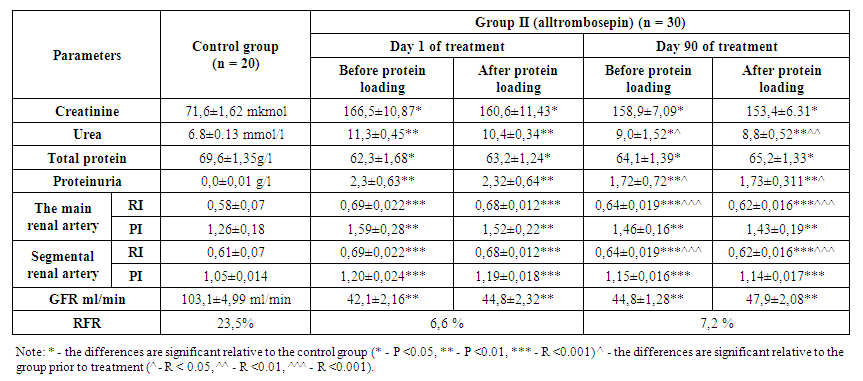 | Table 2. Dynamics of RFR indicators in group II patients |
5. Discussion
- The version of this template is V1. Most of the formatting instructions in this document have been compiled by SAP Productions. SAP Productions offers A4 templates for Microsoft Word. SAP Productions has tried its best efforts to ensure that the templates have the same appearance.SAP Productions permits the distribution and revision of these templates on the condition that SAP Productions is credited in the revised template as follows: “original version of this template was provided by courtesy of SAP Productions”.
6. Conclusions
- In the early stages of chronic kidney disease, it is advisable to monitor the state of the functional reserve of the kidneys.Long-term use of alltrombosepin antiaggregant drug in patients during treatment achieves better preservation of renal functional reserve.In patients with the use of the drug Alltrombosepin, renal functional reserve is well maintained and the progression of chronic kidney disease is slowed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML