Makhkamovа N. E. , Yakubdjanov D. D. , Kxamrayev Y. S.
Department of ENT, Tashkent State Dental Institute, Uzbekistan
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Today, congenital clefts of the soft palate are a fairly common pathology of the dentoalveolar system. Normally, the development of the muscle tissue of the soft palate is completed by 3 years. The poor knowledge of morphology and structural changes in this pathology attracts the attention of many researchers. For this purpose, a study of soft tissues from the area of the former cleft was carried out at various times after the operation. In this work, the muscle tissue, connective tissue, as well as the epithelial cover of the soft palate have been studied. The study was conducted in three age groups. Group 1 - children with congenital cleft up to 3 years old; Group 2 - 6-7 years old and group 3 - 11-12 years old. A light microscope, TEM and SEM were used in the study. Significant destructive and degenerative differences were found in the immediate vicinity of the edge of the existing cleft. In the soft palate, the mucous membrane, in contrast to the hard palate and other parts of the oral cavity, is not associated with bone tissue, but with the movable muscular base of the organ. TEM examination of the muscle base of the soft palate of the control group showed a distinct transverse striation of myocytes. In the first group - children under 3 years of age with congenital cleft of the soft palate, there is a chaotic arrangement of muscle fibers, with an increase in connective tissue layers between them. We also found that morphological differences, especially at the ultrastructural level, increase with age. The most pronounced morphological changes were revealed at the edge of the cleft. We came to the conclusion that if in the first group the changes are moderate in nature without disturbing the general architectonics of the soft palate and are manifested by hydropic disturbances, then in the second and especially in the third group there are pronounced disturbances in the structure of the soft palate.
Keywords:
Congenital cleft of the soft palate, Soft tissues, Degenerative destructive changes, Myocytes, Connective tissue
Cite this paper: Makhkamovа N. E. , Yakubdjanov D. D. , Kxamrayev Y. S. , Structural Features of the Soft Palate Muscles on Congenital Cleft in Different Ages, American Journal of Medicine and Medical Sciences, Vol. 11 No. 3, 2021, pp. 246-252. doi: 10.5923/j.ajmms.20211103.18.
1. Introduction
Congenital cleft of the soft palate is a common pathology of the dento-alveolar system, which is attracted the attention of many researchers. However, the structural features of the soft tissues of this part in this pathology are still poorly understood. There are only a few studies published about this kind of pathology, depending on age. (Khamidov D.Kh 1988).A.Yu. Yuldashev et al. studied ultrastructural features of muscles of the soft palate in congenital cleft before and at various post operation times.Typically, the development of the soft palate muscles is completed by 3 years.According to D.Kh. Khamidova et al. (1988) during this period, the structural differences in muscle tissue in children with developmental defects and in the control group are insignificant. Conversely, with age, morphological differences, especially at the ultrastructural level, worsen. This led the authors to the conclusion that it is preferable to perform surgerical intervention to restore the organ as early as possible, preferably up to three years.A.Yu. Yuldashev et al., having carried out studies of soft tissues from the area of former cleft, at various times after the operation, did not find significant differences in the ultrastructure of the soft palate myocytes.Differences were found only in the immediate vicinity of the edge of the former crevice. They consisted in the thinning of myocytes, their polymorphism, violation of longitudinal orientation, lack of glycogen, degenerative changes in mitochondria. 1.5 - 2 years after early plastic surgery, the soft palate tissue, according to these authors, does not undergo significant changes. Near the edge of the cleft, destructive and degenerative changes are noted.Thus, according to the authors, the production of plastic surgery even at an early age does not lead to the normalization of muscle tissue in children 1.5–2 years after the operation. It is possible that this statement is not entirely true, since the authors did not study the structure of the tissues of the soft palate in children with cleft at different age periods.Some inconsistency of the available data on the ultrastructure of soft palate with a cleft and the result on restoration of the disturbed structure of plastic surgery. Besides, as well as the lack of comprehensive studies using light, scanning and transmission electron microscopy (SEM, TEM), prompted us to study the soft palate with its congenital cleft with using light microscopy, TEM and SEM. At the same time, only muscle tissue was studied, but also connective tissue, as well as epithelial cover of the soft palate in three age groups. Group 1 - children with congenital cleft up to 3 years old; Group 2 - 6-7 years old and group 3 - 11-12 years old.
2. Research Materials and Methods
For transmission electron microscopy (TEM), tissue samples were fixed with 2.5% glutaraldehyde solution in phosphate or cacodylate buffer, after dehydration in alcohol - acetone, they were poured with an epono - araldite mixture. Ultrathin sections obtained with an ultratome «Ultracut» were contrasted in an «Ultrostainer» apparatus and viewed on a «Hitachi H-600» electron microscope.Aimed at scanning electron microscopy, the preparations, after fixation described above, were dehydrated in alcohol-acetone, then dried by the critical point method in the HCP-2 apparatus, and gold was sprayed in an IB-2 apparatus. Examined in a Hitachi S405A electron microscope.The photographs were taken on a Kodak Professional Pro Foto100 or Fujicolor superia 100 color film. The micrographs were scanned on by using Scan Prisa 640P scanner (Acer) and computer processed on a Computek Pentium III Windows 2000 computer.Semi-thin epoxy sections stained with methylene blue – fuchsine were also examined by light optics. Light-optical micrographs were obtained using an Axio scope (Zeizz) microscope with a Sony digital camera.
3. Research Results
In the soft palate, the mucous membrane, in contrast to the hard palate and other parts of the oral cavity, is not associated with bone tissue, but with the movable muscular base of the organ. The oral (anterior part) of the soft palate is covered with stratified squamous non-keratinizing epithelium. The lamina propria of the mucous membrane is rather thin, the bulk of the organ is muscle tissue. The posterior (nasopharyngeal) surface of the soft palate is lined with a single-layer multi-row prismatic ciliated epithelium, similar to the lining of the airways.In the oral mucosa, the most powerful is the spinous layer. The basal layer is represented by one row of rounded oval cells with large nuclei (Fig. 1). In the cells of the spinous layer, there are many tonofilaments and numerous desmosomes, with the help of which the cells are firmly connected to each other (Fig. 1). The granular layer is represented by 1-2 rows of cells. The own connective tissue plate is formed by loose connective tissue.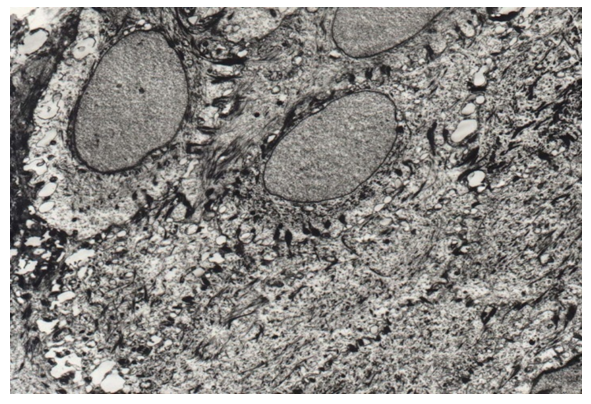 | Figure 1. Tonofilaments and desmosomes in the cells of the basal and spinous layers of the epithelial lining of the soft palate (control). TEM x 4500 |
The most developed muscle base of the soft palate is formed by typical striated muscles, according to the content of myoglobin they can be attributed to white muscles (Fig. 2).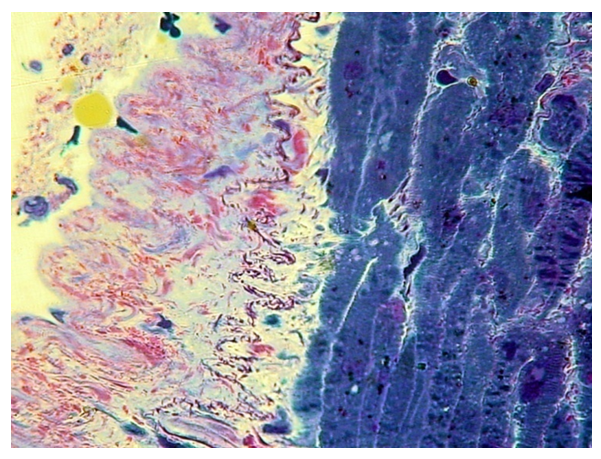 | Figure 2. Connective tissue plate and muscle base of the soft palate (control). PTS 10x20 |
TEM studies of the muscle base of the soft palate of the control group showed a distinct transverse striation of myocytes due to the alternation of light (I) and dark (A) discs, with the corresponding M and H stripes and Z lines. Oval-shaped nuclei with characteristic striation of contours and numerous nuclear pores. Heterochromatin dominates in the nuclei (Fig. 3). | Figure 3. Pronounced transverse striation, correct arrangement of myocytes of the muscle base of the soft palate. (Control). TEM x7500 |
In the first group - children under 3 years old with a congenital cleft of the soft palate, a rather chaotic arrangement of muscle fibers is noted, with an increase in the layers of connective tissue between them - perimisia and endomysia. The epimisium is also well developed (Fig. 4.5). There are quite a few blood vessels in the perimisium. The transverse striation of muscle fibers is well expressed (Fig. 4).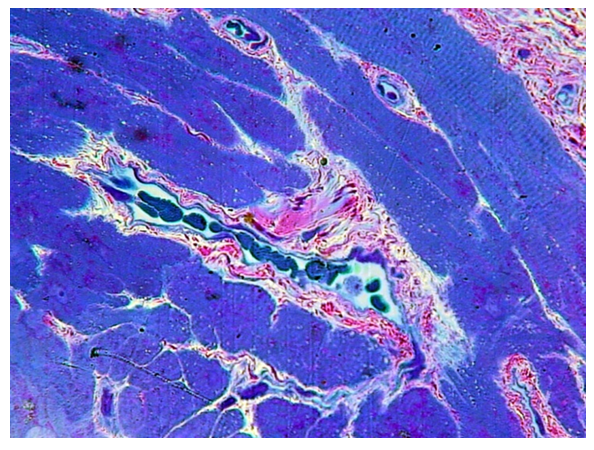 | Figure 4. Overgrowth of perimisia and endomysia in the muscle base of the soft palate with congenital cleft (group I). PTS 10x20 |
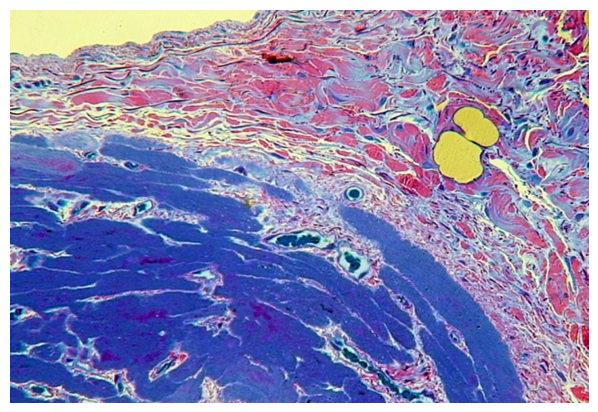 | Figure 5. The proliferation of its own connective tissue lamina of the mucous membrane, perimisia and endomysia of the muscular base of the soft palate in congenital cleft (group I). PTS 10x20 |
TEM studies of tissues of the soft palate in group 1 showed that vacuolization of the cytoplasm takes place in the cells of the spinous layer, a decrease in the content of tonofilaments and the number of desmosomes, the nuclei become smaller in size, and sometimes small nucleoli are determined in them (Fig. 6).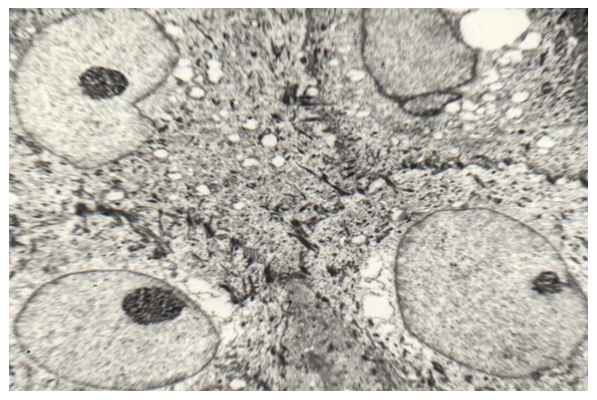 | Figure 6. Coarsening and a decrease in the number of tonofilaments and desmosomes, vacuolization of the cells of the spinous layer of the epithelial lining of the soft palate in congenital cleft (group I). TEM x 3000 |
Myocytes in muscle fibers are not as symmetrical as in the control group. The characteristic striation is preserved, light (I) and dark (A) discs with corresponding M and H stripes and Z lines are clearly defined. Mitochondrias are small and rare. Light spaces are located between myocytes, dilated blood vessels and large vacuoles are defined between the fibers (Fig. 7). This indicates hydropic disturbances in the muscular base of the soft palate.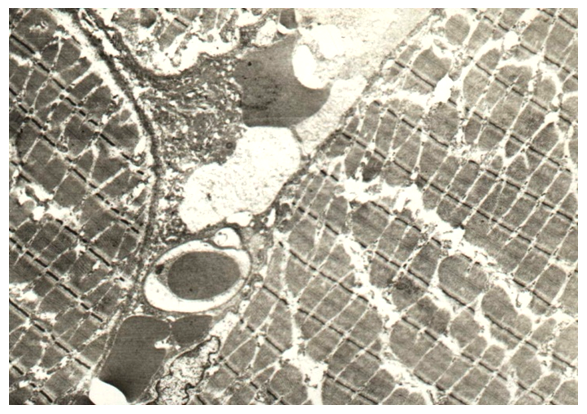 | Figure 7. Edema of interfiber spaces and myocyte sarcoplasm in congenital cleft (group I). TEM x 4500 |
SEM studies have shown thinning of the epithelial lining. The appearance between the epithelium and its own connective tissue lamina of various sizes of lacunae (Fig. 8). The connective tissue plate of the mucous membrane itself thickens, its fibers become coarse. On the border of its own connective tissue plate and the muscular base, the presence of large lacunae is also noted.  | Figure 8. Thinning of the epithelial lining, coarsening of the fibers of its own connective tissue plate, the appearance of lacunae in congenital cleft (group I). SEM x 1000 |
In the second group - children with a cleft 6–7 years old, the structural changes of the soft palate are more pronounced. Muscle fibers are of different sizes and shapes. They are located rather chaotic. In the layers of perimisium and endomisium, dilated microvessels with the phenomenon of stasis and sludge of erythrocytes (Fig. 9).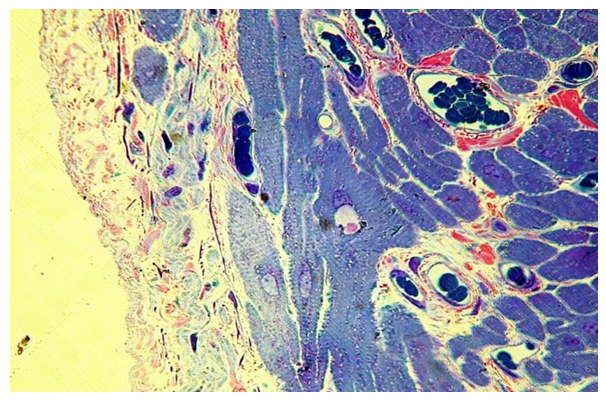 | Figure 9. The proliferation of the own connective tissue lamina of the mucous membrane, perimisia and endomysia of the muscular base of the soft palate, stasis and sludge of erythrocytes in microvessels with congenital cleft (group II). PTS 10x20 |
In some areas, especially near the cleft, the proliferation of connective tissue leads to the splitting of muscle fibers into separate myocytes, which retain their transverse striation (Fig. 10). In such areas, dilated veins and venules are determined. In the lumen of which the blood cells are not determined.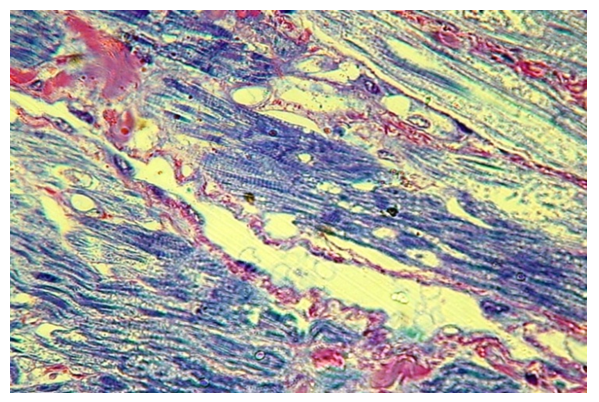 | Figure 10. The proliferation of its own connective tissue by the muscular base of the soft palate at the edge of the cleft (group II) / PTS 10x20 |
TEM studies have shown that in the cells of the spinous layer of the epithelial lining, the number of vacuoles increases, the tonofilament becomes smaller, and they become coarser, and the number of desmosomes also decreases (Fig. 11).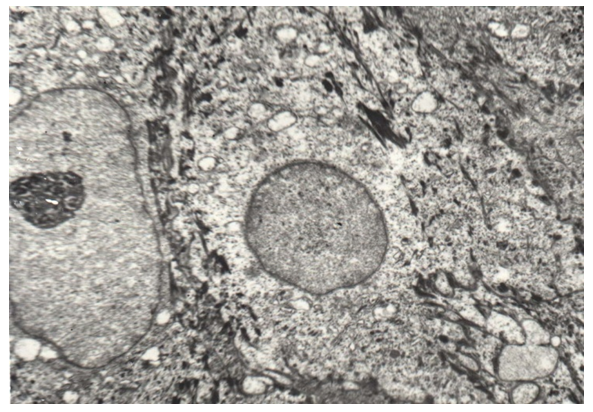 | Figure 11. Coarsening and a decrease in the number of tonofilaments and desmosomes, vacuolization of cells of the spinous layer of the epithelial lining on soft palate in congenital cleft (group II). TEM x 3000 |
In the muscle base, myocytes retain a characteristic transverse striation, depending on the severity of light (I) and dark (A) discs with M and H stripes and Z lines. In the interfiber spaces (endomysium), along with capillaries with dilated lumens, areas of hemorrhage are determined (Fig. 12). In zones with the most chaotic arrangement of fibers, the characteristic striation is broken in places (Fig. 13). The Mitochondria are swollen with cleaned matrix, intercalated discs are compacted (Fig. 13).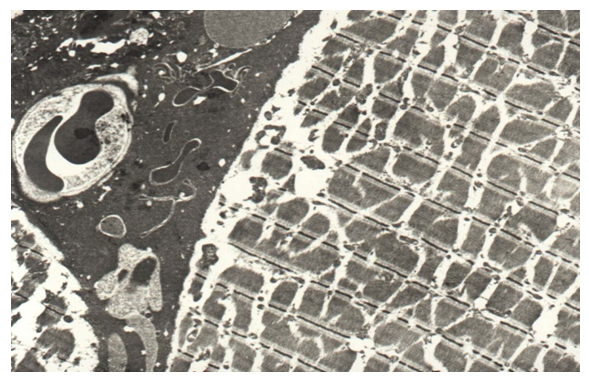 | Figure 12. Edema of interfiber spaces and sarcoplasm of myocytes, the appearance of free erythrocytes in congenital cleft (group II). TEM x 4500 |
 | Figure 13. Edema and vacuolization of myocyte sarcoplasm, disturbance of disc structure in congenital cleft (group II) / TEM x 4500 |
SEM studies have shown that, as in the first group, there is an effusion of the epithelial lining, coarsening of the fibers of the own connective tissue plate of the soft palate (Fig. 14). | Figure 14. Significant thinning of the epithelial lining, coarsening of the fibers of its own connective tissue plate, the appearance of lacunae in congenital cleft (group II). SEM x 1000 |
In the third group - children with a cleft 11-12 years old, the structural changes of the soft palate are most pronounced.The cells of the basal and spinous layer of the epithelial lining lose their tonofilaments, little becomes desmosome, and pathological mitoses occur in the basal layer (Fig. 15). Heterochomatin dominates in the nuclei.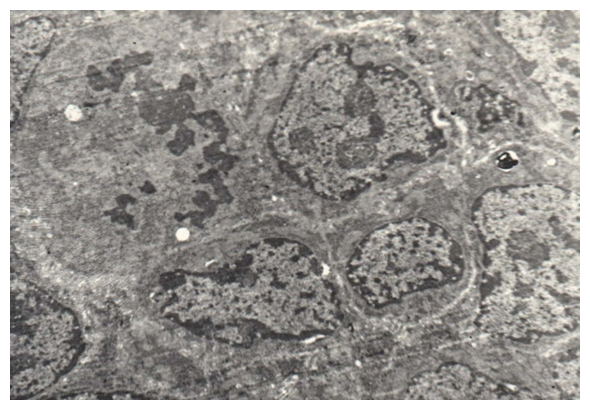 | Figure 15. Disappearance of tonofilaments and desmosomes, pathological mitoses of the cells of the basal layer, enlargement of nuclei in congenital cleft (Group III). TEM x 3000 |
These ultrastructural changes may indicate the presence of dysplasia in the epithelial lining.In the muscle base of the soft palate in this group, the changes are most pronounced. Muscle fibers have different shapes and sizes, on semi-thin sections, the transverse striation is poorly expressed, the appearance of small vacuoles in the cytoplasm of myocytes is noted. In microvessels, erythrocyte sludge takes place (Fig. 16).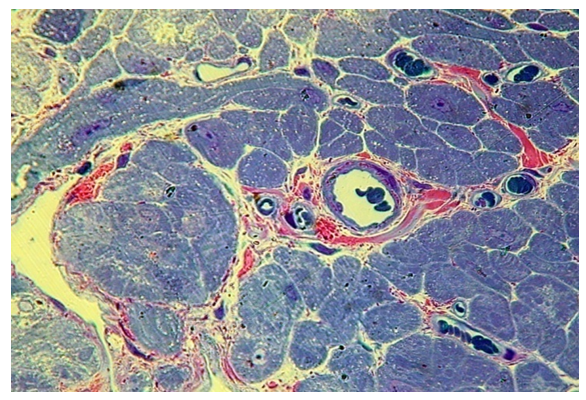 | Figure 16. Chaotic arrangement of fibers, proliferation, perimisia and endomysia, stasis and sludge of erythrocytes in microvessels with congenital cleft (group III). PTS 10x20 |
At the edge of the cleft, the changes, as well as in the second group, are most pronounced. The proliferation of connective tissue leads to stratification of muscle fibers, and individual myocytes appear to be walled up in the overgrown connective tissue. Harewith, the transverse striation of myocytes is preserved. (fig. 17).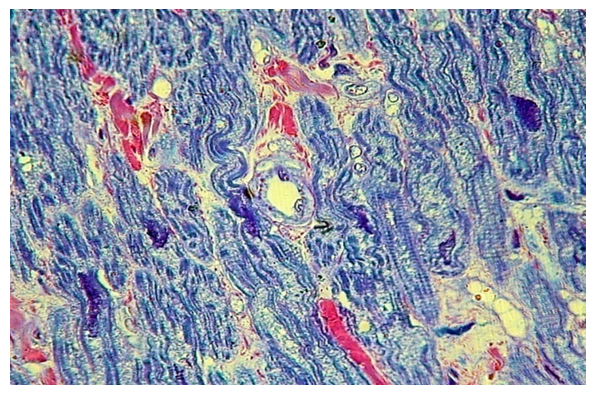 | Figure 17. Pronounced growth of its own connective tissue with the disintegration of the fibers of the muscle base of the soft palate at the edge of the cleft (group III). PTS 10x20 |
TEM studies have shown that along with edema and swelling of mitochondria, there is also edema of myocyte sarcoplasm, disruption of the structure of discs and stripes. This is combined with perinuclear edema (Fig. 18). There are also intercellular edema with a free arrangement between erythrocyte myocytes (Fig. 19).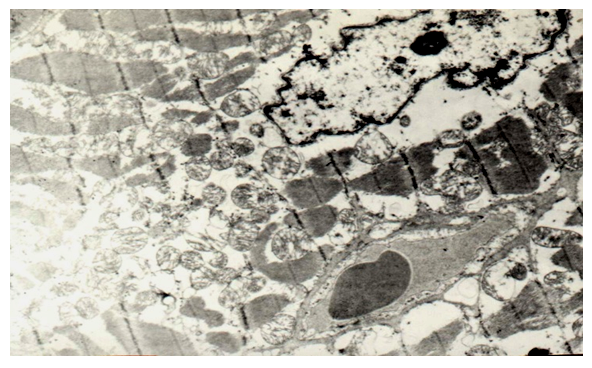 | Figure 18. Perinuclear edema and vacuolization of myocyte sarcoplasm, disruption of the disc structure, swelling of mitochondria in congenital cleft (group III). TEM x 7500 |
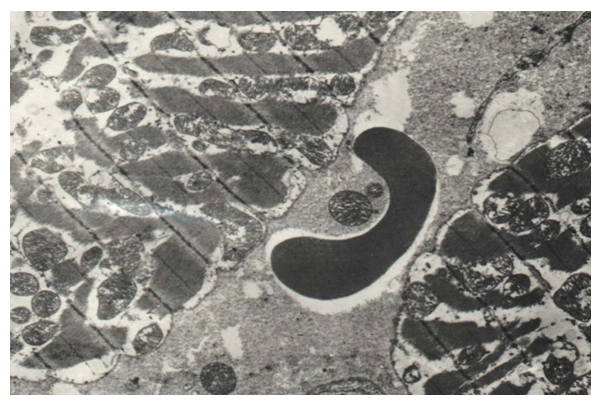 | Figure 19. Interfibre edema, vacuolization of myocyte sarcoplasm, disruption of disc structure, swelling of mitochondria in congenital cleft (III group) / TEM x 7500 |
SEM studies also showed a chaotic arrangement of myocytes of the muscle base of the soft palate with the formation of large intercellular spaces, in which individual blood cells are determined (Fig. 20).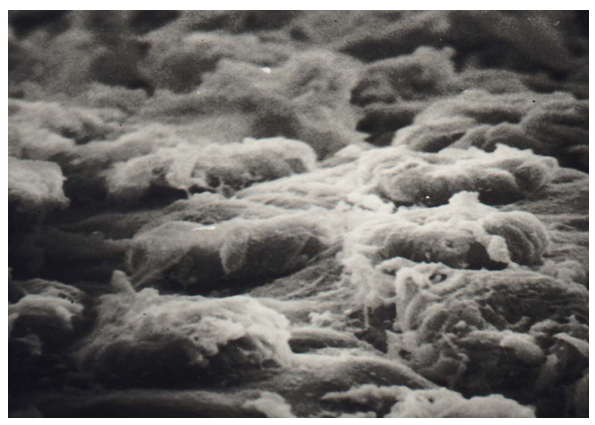 | Figure 20. Disintegration of myocytes of the muscular base of the soft palate in congenital cleft (Group III). SEM x 1500 |
Studies have shown significant violations of the structure of the soft palate in all three groups of children with congenital cleft.If in the first group the changes are moderate in nature without disrupting the general architectonics of the soft palate and appear, mainly in hydropic disturbances, then in the second and especially in the third group, pronounced disturbances in the structure of the soft palate are noted.In the epithelial lining in the third group, the changes are dysplasia. In myocytes, pronounced randomness is combined with degenerative changes in myocytes and proliferation of connective tissue in the cleft zone, which reaches significant severity in the third group. The morphological picture indicates sometimes irreversible changes in the mucous membrane and muscle base.
4. Conclusions
Pathomorphological structural rearrangements of the muscle tissue of the soft palate in children with congenital cleft palate insignificant in the early stages of postnatal life. The aggravation of these processes is correlated with the age of the child.
5. Practical Recommendations
• Our observations allow us to assert that it is advisable to carry out surgical intervention in the early period, when structural changes are minimal.• In the third age group, for the plastic of the palate, it is necessary to excision of irreversibly altered tissues in the cleft zone.
References
| [1] | Strong E B Buckmiller L M Management of the cleft palate Facial Plast Surg Clin North Am 20019115–25. |
| [2] | Vanderas A P. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987; 24(3): 216–225. |
| [3] | Shprintzen R J, Siegel-Sadewitz V L, Amato J, Goldberg R B. Anomalies associated with cleft lip, cleft palate, or both. Am J Med Genet. 1985; 20(4): 585–595. |
| [4] | Fraser F C. The genetics of cleft lip and cleft palate. Am J Hum Genet. 1970; 22(3): 336–352. |
| [5] | Lorente C, Cordier S, Goujard J. et al. Occupational Exposure and Congenital Malformation Working Group. Tobacco and alcohol use during pregnancy and risk of oral clefts. Am J Public Health. 2000; 90(3): 415–419. |
| [6] | Källén B. Maternal drug use and infant cleft lip/palate with special reference to corticoids. Cleft Palate Craniofac J. 2003; 40(6): 624–628. |
| [7] | Hopper R A, Cutting C, Grayson B. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. Cleft lip and palate; pp. 201–225. |
| [8] | Fisher D M, Sommerlad B C. Cleft lip, cleft palate, and velopharyngeal insufficiency. Plast Reconstr Surg. 2011; 128(4): 342e–360e. |
| [9] | Bessell A, Hooper L, Shaw W C, Reilly S, Reid J, Glenny A M. Feeding interventions for growth and development in infants with cleft lip, cleft palate or cleft lip and palate. Cochrane Database Syst Rev. 2011; (2): CD003315. |
| [10] | D'Antonio L L, Davio M, Zoller K, Punjabi A, Hardesty R A. Results of Furlow Z-plasty in patients with velocardiofacial syndrome. Plast Reconstr Surg. 2001; 107(4): 1077–1079. |



















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

