-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(3): 219-223
doi:10.5923/j.ajmms.20211103.12
Received: Mar. 3, 2021; Accepted: Mar. 26, 2021; Published: Mar. 28, 2021

Study of Antiexudative Effect of Gel Containing Extract of Convolvulus Arvensis
Z. Z. Khakimov1, A. Kh. Rakhmanov1, U. B. Yakubova2, K. Sh. Shukurlaev2
1Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan
2Urgench Branch of the Tashkent Medical Academy, Uzbekistan
Correspondence to: A. Kh. Rakhmanov, Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The antiexudative effect of a gel containing Convolvulus arvensis extract was studied in an experiment on male white rats. The mechanism of the antiflogogenic action of this compound is associated with its antihistamine and antiseratonin action. It is concluded that the pharmacological activity of the gel containing Convolvulus arvensis extract practically does not differ from the ibuprofen gel.
Keywords: Inflammation, Local action, Exudation
Cite this paper: Z. Z. Khakimov, A. Kh. Rakhmanov, U. B. Yakubova, K. Sh. Shukurlaev, Study of Antiexudative Effect of Gel Containing Extract of Convolvulus Arvensis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 3, 2021, pp. 219-223. doi: 10.5923/j.ajmms.20211103.12.
Article Outline
1. Introduction
- The development and implementation of effective import-substituting anti-inflammatory drugs from local raw materials is one of the important areas of modern pharmacology, because they are widely used in the treatment of diseases in the pathogenesis of which inflammation takes the leading place. Non-steroidal anti-inflammatory drugs (NSADs) are widely used in daily life. However, the regular use of the latter does not always provide the necessary therapeutic effect, moreover, they quite often cause side effects (gastro-, nephro-, cardio-, hemato-, hepatotoxicity), which is associated with their ability to penetrate the histohematogenous barriers and the manifestation of systemic action [1,2,3]. Therefore, in recent years, great importance has been attached to the local use of anti-inflammatory drugs in soft drug forms [4,5,6,7]. It is known that aqueous extract of the aerial part of Convolvulus arvensis has a distinct anti-inflammatory effect in experimental animals at oral administration, which is associated with its antagonism in relation to inflammatory mediators, a decrease of vascular permeability and suppression of hyaluronidase activity [8]. One of the effective ways to prevent the development of adverse drug reactions is their local application in the form of a gel, cream, ointment, etc. Moreover, their action is limited to the place of their application and does not allow the development of systemic action. Gels, due to the special composition of excipients, promotes the fast onset of the local anti-inflammatory effect [9,10]. Therefore, gels are currently the most common "modification" of NSAIDs for external use.The above circumstance served as the basis for the development of a new drug form - a gel containing an extract of Convolvulus arvensis and the study of its antiexudative activity at local application.
2. Material and Methods
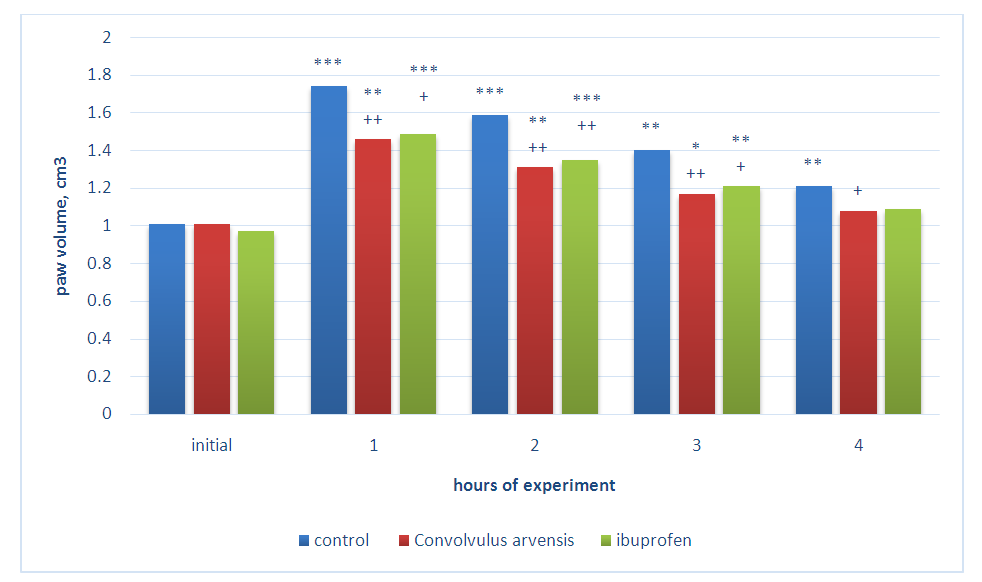
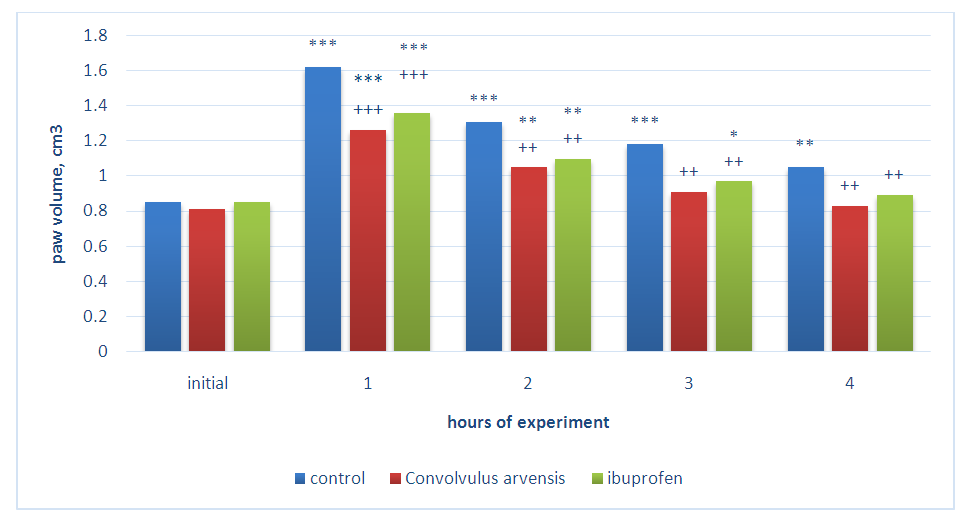
- Experimental studies were carried out on sexually mature white male rats with an initial weight of 155-180 g. Before the experiment, the animals were quarantined for 12-14 days. All animals were kept in vivarium conditions (with natural lighting, at a temperature 22-24°C; relative humidity 40-50%) using a standard diet. Each experimental group consisted 6 individuals. The antiexudative effect of the drugs was studied on models of acute inflammatory edema of the paws of animals by subcutaneous injection of 6% dextran solution, 0.2% histamine solution and 0.01% serotonin solutions (0.1 ml per animal) into the paw of the hind right limb of the rats. These models of inflammatory edema are widely used to assess the anti-inflammatory activity of new potential drugs [11,12,13]. Measurement of the volume of the paws of animals was carried out by the oncometric method using a plethysmometer [14] before and hourly for four hours after the injection of the flogogens. The value of anti-inflammatory activity (VAA) of drugs was calculated according to the formula VAA = Vcontrol-Vexperiment / Vcontrol x 100 =% [11]. A gel containing Convolvulus arvensis extract and ibuprofen gel was applied to the surface of the right hind paw of the animals one hour before flogogen injection and after each measurement. Experimental studies were carried out in accordance with the rules of good laboratory practice (GLP) in conducting preclinical studies in the Republic of Uzbekistan.The approval of the Ethic Committee of Uzbekistan were taken before carrying out the experiments on animals. All experiments were performed in compliance with the requirements of the European Convention "On Protection of vertebrate animals used for experimental and other scientific purposes" (Strasbourg 1986).
2.1. Statistical Analysis
- The received results were subjected to the statistic processing with the using of standard software package Biostat 2009 on well-known method of variation statistics, all results were considered as mean ± SEM. Differences between groups were analyzed using the Student’s t-test. P<0.05 was considered significant.
3. Results and Discussion
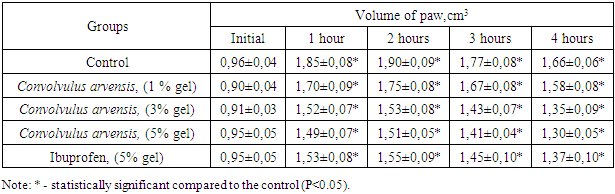
- The results of the studies showed that the subplantar injection of the dextran solution leads to increase of the volume of paws an almost two times during the first two hours from the beginning of the experiment, which remained without significant changes in the next four hours of the experiment. This result once again convincingly proves the high activity of dextran as a flogogen. The use of 5% ibuprofen gel led to a distinct decrease of the level of paw’s edema of rats developing under the influence of dextran. So, after 1 hour the paw of animals increased by 61.0%, after 2 hours - 63.0%, and after 3 and 4 hours - 52.6% and 44.2% in comparison with the initial volume. During the indicated study periods, the VAA of the drug was 34.8; 36.2; 38.3 and 40.0%, respectively. It can be seen that the ibuprofen gel, when applied locally, exhibits a distinct anti-inflammatory activity. Further studies have shown that the gel containing Convolvulus arvensis extract also has anti-exudative activity. It can be seen in table 1 that during the application of 1% gel of the Convolvulus arvensis extract, VAA was 10.1 and 9.6% at the first two hours of the experiment. The increase of the concentration of the gel to 3% led to rise degree of antiexudative effect of Convolvulus arvensis extract. So, after 1 hour from the onset of dextran action, the volume of the paws increased by 67.0%, and after 2 hours - 68.1%. This effect persisted with slight fluctuations in subsequent periods of observation. The calculation of the VAA during the using of 3% gel showed that VAA was equal to 31.4; 34.0; 35.8 and 37.1%, respectively in the indicated periods of the experiment.
|
4. Conclusions
- 1. The gel containing Convolvulus arvensis extract exhibits a distinct anti-exudative effect on the model of dextran-induced aseptic arthritis.2. The anti-exudative effect of the gel containing the extract of Convolvulus arvensis is largely due to the antagonistic effect on the histamine and serotonin systems.3. In terms of its pharmacological activity, the gel containing Convolvulus arvensis extract practically does not differ from the activity of ibuprofen gel.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML