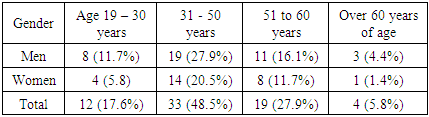Iminov B. M.1, Iriskulov B. U.2, Kodirov Sh. N.1, Kodirov M. Sh.1
1Department «General Surgery», Andijan State Medical Institute, Andijan, Uzbekistan
2Department of Normal and Pathological Physiology, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Iminov B. M., Department «General Surgery», Andijan State Medical Institute, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article examined 68 patients with chronic gastric ulcers and duodenum with complicated anaemia, who were re-hospitalized one month after stomach resection according to B-I and B-II methods. The serum content of these patients was reported to have been compared with pre-operative data after 1, 3 and 6 hours of iron and hydroionized iron injection.
Keywords:
Iron deficiency, Chronic anaemia, Ulcers
Cite this paper: Iminov B. M., Iriskulov B. U., Kodirov Sh. N., Kodirov M. Sh., Consequences after the Resection of the Stomach and Duodenum with Chronic Anaemia, American Journal of Medicine and Medical Sciences, Vol. 11 No. 2, 2021, pp. 91-94. doi: 10.5923/j.ajmms.20211102.04.
1. Relevance
The importance of glands in humans and animals is enormous. Among them, the role of enzymes produced by the glands of the gastrointestinal tract, involved in the absorption of iron is significantly greater. Chronic gastrointestinal diseases such as gastritis, stomach mucosa atrophy, iron deficiency and cyanocbolamine leads to anaemia of different origins. Anemia can also be the first sign of other diseases or arises as a complication. At the same time, people with lifestyle disorders can cause up to severe diseases (Kushpir I.E., 2009; Loginov V.A., 2020).In the early 20s of the last century began to appear the articles about anemia that occurs in the period after resection of the stomach. Many scientists have discovered hyperchromic anemia in their patients and called it Anaemia perniciosa. But this disease did not pass after liver treatment, causing from hyperchromic to hypochromic anemia. In the 1930s, there was a huge amount of evidence of hypochromic anemia that arose after a stomach resection, which Gordon Taylor first noted in his research. Surgical treatment of ulcers of the stomach and duodenum is the main treatment not only in our country but also in other foreign countries. The method of operative treatment is stomach resection. Significant attention is given to the chronic anaemia as the post-operative complications of this operation. The physiological structure of the gastrointestinal tract (GIT) is not restored after its breakdown of cell structure. Therefore, atrophic inflammation develops in the epithelial part of the GIT and vitamin absorption is disturbed. (Karimov H.E. with coauthors, 2017., Zubarev P.N. 2004, Chissov V.I., Alexandrova L.M. 2010). Iron is mainly absorbed in the duodenum and in the small intestine, and must be ionized during absorption. A study on the process of absorption of iron preparations has been under way for many years. During the study, it was proved that ionized iron is absorbed in the stomach and in the initial part of the duodenum. Once the stomach is resected, the process of absorbing iron into the GIT is disturbed. The disturbance of iron absorption in the stomach and duodenum in the human body is affected by the removal of the part of the stomach and duodenum. At the same time, the surface where the iron is absorbed is also reduced. So, in the GIT the formation of enzymes in quality and quantity reduces. In many cases, under the Bilroth II method, the enzymes involved in the iron absorption process are not involved in food digestion. (Chernousov A.F., 2004; Akhrorov B.M. with coauthors, 2011., Khodzhibaev A.M. with coauthors, 2011). This condition adversely affects the patient. Therefore, it is necessary to study this absorption process and carry out the necessary treatment measures. Because chronic anemia after resection leads to polyorganic dystrophic changes.
2. Materials and Methods of Research
We selected 68 patients who had been treated for stomach and duodenum diseases for long period and had chronic iron-deficiency anaemia, between 2015 and 2020, and who were resected using different techniques. These patients consisted of 27 women (39.7 percent of the total) and 41 men (60.3 percent) between the ages of 21 and 69. These patients were hospitalized and examined for rehabilitation one month after the operation.
3. Research Results
The examination included a general blood test, the condition of gastric mucosa during fibrogastroduodenoscopy (FGDS), iron quantity and functional status, clinical anastomosis, food evacuation, X-rays and a iron test in serum. Cases of haemorrhaging from anastomosis and other causes in patients were not included in the clinical studies.Table 1. Distribution of patients by age and gender
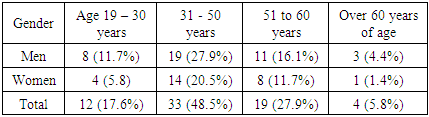 |
| |
|
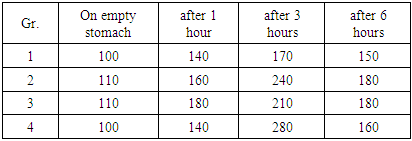
We took 8-10 mg of blood from the patient in the morning, and then we gave the patient 1.0 ml of an iron supplement saturated with hydrogen. The patient then took in 30 ml 3% hydrogen chloride. Blood was drawn from the vein after 1, 3, 6 hours after intake. After that, the patient was allowed to eat. The blood was stored in a thermostat at 4-6 degrees, centrifuged after 2 hours at 2,000 rpm/min, and thus plasma was separated.The resulting plasma was again centrifuged at 2000 o/min to separate the plasma. The resulting plasma was tested for iron content. Such examinations were performed on patients before and after the operation.Depending on the operation performed, the patients were divided into two groups: 56 patients who had 2/3 of their stomach removed after B-I resection; 6 patients who have undergone a resection ¾ part of the stomach due to gastroptosis caused by decompensated stenosis. These 62 patients are divided into three subgroups.The first subgroup includes 31 patients with an iron concentration of up to 60% after the first hour; 20 patients with concentrations between 61% and 150% into subgroup 2; 11 patients in subgroup 3 with concentrations above 151%. The 6 patients who have undergone B-II resection constitute separately the 4th subgroup. The patients of this group were put through the anterior part of the colon with a long loop with gastroenterosomes of the Braun bridge, 2/3 part of the stomach resected and duodenum was left at the beginning of the intestinal obstruction. All patients participating in the survey were selected on the basis of the examinations, as described above, even in the pre-operative period.After being saturated with iron, we preferred to check the absorbed iron graphically to facilitate generalization (post treatment curves). It is possible to look at each case separately and to compare whether it has changed in one way or another. This can be understood as a comparison of the concentration of iron in the blood serum at a given point in time in percentage (in 1.3.6 hours) in the pre-operative and post-operative periods.For example, if we take the concentration on an empty stomach before saturation - 100%, then in one hour - 140%, in three hours - 170%, in six hours - 150%, our highest indicator is - 170%. This indicator is relative and is the average indicator of all patients. In two patients, the indicator was 110% on an empty stomach, 160% within an hour after the introduction of ionized divalent iron, 240% after three hours, 180% after 6 hours, on the third patient on an empty stomach 140%, 180% after an hour after the introduction of ionized divalent iron. 210% after in three hours, 180% after six hours. This shows that all three patients had high iron resorption after three hours - 150%, and iron absorption variability was detected.These figures are shown in table 2.Table 2. Pre-operative indicators of patients after intake of ionized iron preparations
 |
| |
|
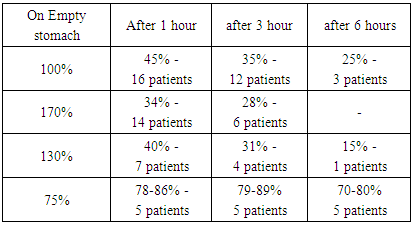
In the first group post-operative iron absorption is declined by an average of 45% - three times in 16 patients, 35% - four times in 12 patients and 25% - six times in 3 patients. In the second group, the average iron absorption was 170%, in 14 patients after surgery - 34%, that is, five times, and in 6 patients 28%, six times less. The third group showed a reduction of absorption by 40% - 7 times in four patients - 4, 31% - 5 times in four patients, and 10 times less in one patient - a very small number of 15%. Table 3. Post-operative indicators of patients after intake of ionized iron preparations
 |
| |
|
All six patients who were resected by B-II method came with post-operative anaemia. When we examined them with the method described above, the amount of iron in the blood plasma was 70-80% on an empty stomach, which is 2-3 times lower than normal. In the study of ionized divalent iron in 1 hour after intake, 4 patients had 78-86%, after 3 hours the figure remains unchanged at 79-87% and after 6 hours 70-80%. In one patient, the baseline level was 66 per cent and did not change after saturated iron treatment.In connection with the above, in addition to the post-operative examinations described above, we have also studied changes in the rest of the stomach cavity by conducting our FGDS patients. In the first group of patients in the post-resection period, there were 19 patients with superficial gastritis and anastomositis, one patient had symptoms of total anastomositis, and the rest had no changes. In the second group there were 17 patients with superficial gastritis and anastomositis, one patient developed anastomositis, the other two patients had no changes. In the third group, eight patients had superficial gastritis and anastomositis, one patient had symptoms of extensive anastomositis, and the other two patients had no changes. All patients preserved anastomosis permeability and food evacuation was not disturbed, which was revealed on contrast X-rays of patients.In the fourth group, symptoms of superficial gastritis and anastomositis were detected in 5 patients who were resected with the B-II method, evacuation maintained. In one patient, the process of iron absorption was completely disrupted due to the flattening of the gastric mucosa and a reduction in the number of glands. The patient, due to a decrease in acid secretion in the pH meter, the amount of acid decreased, and therefore the formation of divalent iron was severely impaired. which led to the development of severe iron deficiency anemia. In addition, in patients the rapid evacuation of food leads to a decrease in the absorption of not only iron, but also B12 vitamin and folic acid.Symptoms of superficial gastritis and anastomositis were found in 5 patients who underwent B-II resection, and food evacuation is preserved. In one patient, the process of iron absorption was completely disrupted due to the flattening of the mucous membrane of the stomach and gland contraction. All 68 patients were biopsied from several places to determine the state of their glands in the postoperative cavity, and when the relative number of glands and the state of secretion were determined, it turned out that the number of glands was significantly reduced.Studies have shown that in 68 patients who received ionized divalent iron, the absorption of the drug worsened after stomach resection rather than before surgery. In 48 patients functional condition of iron-sucking gastric glands worsened 3-5 times. In 5 main group patients and in patients who underwent stomach resection according to the B-II method, after the introduction of ionized divalent iron completely reduced the absorption of iron, and the dynamics of iron in plasma decreased by 5-6 times. By this time, it was found that the plasma level of iron had dropped by 22-26% from the initial level.It should be noted that in a healthy person iron is absorbed in the stomach, duodenum and in the upper part of the small intestine. The lower you go into the small intestine, the less iron is absorbed.In addition, many scientists have shown that food-borne trivalent iron begins to be absorbed when it is altered by hydrogen chloride in the stomach. This process is disrupted by stomach resection. If the stomach is cut by the B-II method, despite what modification, the food does not transfer from the duodenum to the small intestine, but instead goes directly into the lower part of the small intestine.This condition leads to a reduction in the surface of iron absorption, which can lead to severe anaemia because the iron does not pass through the main absorption pathways due to the disruption of the divalent exchange. Although the compensatory function of the body works, it takes some time for the body to fully absorb iron. Based on the results of the above study and sources in the literature, it has been determined that iron deficiency anaemia occurs in 72-76% of patients in the post-operative period. The reasons were as follows:After resection of the stomach and duodenum due to inflammation of the rest of the stomach, there is a decrease or complete disturbance of the secretory function of the stomach. This condition leads to disturbance of absorption of iron, vitamin B12 and folic acid, which leads to a decrease of the absorbing volume of the rest of the stomach and an accelerated excretion of bile and a change in the volume of food.
4. Conclusions
1. After the resection of the ulcer of the duodenum and stomach (regardless of which modification of the method) patients have serious iron absorption disorders. Iron intake disorders occur in 72-76% of patients and increase 3-5 times after surgery.2. One of the severe complications after resection of gastric ulcer and duodenal ulcer is the occurrence of a state of hypotheremia. This leads to depletion of iron stores, despite the fact that iron is absorbed.3. Meanwhile, patients develop hypochromic agastral anemia, which, in case of belated treatment, leads to deep multiple organ failures in the body.
References
| [1] | B.M. Akhrarov, A.T. Sultonov, A.F. Rasulov, U.K. Syrjiddinov, A.B. Ortikov “Clinical justification for the prevention of recurrence of haemorrhagic complications of ulcerative disease of the duodenum” II Congress of the Association of Emergency Medical Doctors. Tashkent 2011. p.133. |
| [2] | G.K. Zherlov, A.P. Koshel, A.V. Maksimov, V.S. Agadzhanov. Ways to improve the quality of life of patients after gastrectomy and subtotal distal gastrectomy. // Russian journal of gastroenterology, hepatology, colo-proctology. 2000. - No. 3. p. 82-85. |
| [3] | P.N. Zubarev “Post-resection and post-gastric diseases” Practical oncology. - 2001. 3. p. 31-34. |
| [4] | A.M. Hadjibaev, I.V. Melnik, A.B. Yeshmuratov “Medical and diagnostic tactics for acute gastroduodenal ulcers” II Congress of the Association of Emergency Medical Doctors. Tashkent 2011. p.362. |
| [5] | G.G. Sadigova Anemia in inflammatory intestinal diseases. // Coloproctology. - 2016. –no.3. p. 84-90. |
| [6] | G.C. Guidi, C.L. Santonastaso “Advancements in anemias related to chronic conditions” ClinChem Lab Med 2010; 48(9): 1217-26. |
| [7] | A. Zhu, M. Kaneshiro, J.D. Kaunitz “Evaluation and Treatment of Iron Deficiency Anemia” A Gastroenterological Perspective. Dig. Dis. Sci. 2010; 55:548-59. |
| [8] | E. Lahner, B. Annibale “Pernicious anemia: new insights from a gastroenterological point of view” World Journal of Gastroenterology. 2009 Nov. 7; 15(41): 5121-8. |
| [9] | R.W. Malizia, B.M. Baumann, M.E. Chansky, et al. “Ambulatory dysfunction due to unrecognized pernicious anemia” J. Emerg. Med. 2010 Apr; 38(3): 302-7. Epub, 2007, Dec 3. |
| [10] | M.R. Turner, K. Talbot “Functional vitamin B12 deficiency” Pract. Neurol. 2009 Feb; 9(1): 37-41. |
| [11] | L.T. Vlasveld “Low cobalamin (vitamin B12) levels in multiple myeloma: a retrospective study” Neth. J. Med. 2003 Aug; 61(8): 249-52. |
| [12] | S. Adachi, T. Kawamoto, M. Otsuka, T. Todoroki, K. Fukao. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency Ann. Surg., 232 (2) (2000), p. 199. |
| [13] | K.W. Seo. Iron deficiency anemia after bariatric surgery J. Metab. Bariatr. Surg., 3 (2) (2014), p. 33-37. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML