-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(1): 12-14
doi:10.5923/j.ajmms.20211101.03
Received: Nov. 2, 2020; Accepted: Dec. 11, 2020; Published: Dec. 28, 2020

Amitriptyline in the Treatment of Early Carpal Tunnel Syndrome
Shah TA1, Wazib A.2, Rahman S.2, Kamal FN3
1Department of Medicine, Uttara Adhunik Medical College, Dhaka, Bangladesh
2Department of Medicine, Enam Medical College, Dhaka, Bangladesh
3Department of Medicine, Delta Medical College, Dhaka, Bangladesh
Correspondence to: Shah TA, Department of Medicine, Uttara Adhunik Medical College, Dhaka, Bangladesh.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Early carpal tunnel syndrome is treated conservatively with weight reduction and symptomatic treatment for sensory complaints. Amitriptyline is a cheap and widely used drug for neuropathic symptoms. This randomized control trial on 80 cases showed that Amitriptyline was superior to placebo in reducing the sensory symptoms of early carpal tunnel syndrome.
Keywords: Amitriptyline, Early carpal tunnel syndrome
Cite this paper: Shah TA, Wazib A., Rahman S., Kamal FN, Amitriptyline in the Treatment of Early Carpal Tunnel Syndrome, American Journal of Medicine and Medical Sciences, Vol. 11 No. 1, 2021, pp. 12-14. doi: 10.5923/j.ajmms.20211101.03.
1. Introduction
- Compression of the median nerve at the level of the carpal tunnel gives rise to signs and symptoms of carpal tunnel syndrome (CTS) [1]. CTS comprise 80-90 percent of all entrapment neuropathies [2]. The exact prevalence is not known, but assumed to be in 1-3.5/100000 range in the subcontinent [3]. The exact cause of CTS is unclear, and hence, it is related to numerous risk factors like pregnancy; occupations involving exposure of the hand and wrist to a high pressure, a high force, repetitive work and vibrating tools and systemic co-morbidities like obesity, thyroid dysfunction, diabetes mellitus, and rheumatoid arthritis [4]. The most common symptoms of CTS are sensory ones – tingling, numbness and pain in hand. These symptoms can occur at any stage of CTS [5]. They are more troublesome at night causing sleep disturbance. The quality of life is hampered significantly among the sufferers [6].Carpal tunnel syndrome is classified into early, moderate and severe stages, according to severity in nerve conduction study (NCS) [7]. Early CTS has normal routine NCS of median nerve but a relative prolongation of median sensory latency by ≥0.5 ms in median versus ulnar sensory comparison study. Moderate CTS is the one with prolonged median sensory latency and/or distal motor latency. CTS is classified as severe when sensory and/or motor action potentials are reduced (SNAP and CMAP, respectively). Surgical decompression is indicated in severe CTS and also considered in moderate CTS when conservative treatment fails [8]. Early CTS cases are treated conservatively with advice for weight reduction and symptomatic treatment for sensory complaints [9]. Antiepileptic drugs, tricyclic antidepressants (TCA) and selective serotonin receptor blockers are widely used for symptomatic relief in CTS [10]. Amitriptyline is the cheapest and most prescribed TCA worldwide for depressive disorders, somatoform disorders and neuropathic symptoms [11]. This study was aimed to assess the efficacy of Amitriptyline in relieving the sensory symptoms of early CTS.
2. Methodology
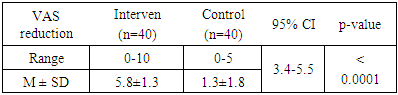
- This randomized control trial was conducted in the medicine outpatient of a tertiary level teaching hospital on 80 patients of early carpal tunnel syndrome (CTS) with sensory symptoms. Diagnosis was confirmed with nerve conduction study. Early CTS was defined as relative prolongation of median sensory latency by ≥0.5 milliseconds in median versus ulnar sensory comparison study, and normal routine median sensory and motor parameters. 40 patients were randomly selected as the intervention group and were prescribed with Amitriptyline. The other 40 patients were taken as control group and were given a placebo. Amitriptyline was given as 10 mg at night for the initial 2 weeks. Dose was increased to 25 mg at night thereafter in case of inadequate response. The sensory symptoms were evaluated with a visual analogue scale (VAS) of 0-10 points subjectively by the patient, at the first visit and then after 2 weeks and 4 weeks. Reduction of score after 4 weeks was compared between the two groups using unpaired T-test.Formal approval was taken from the ethical review committee of the hospital prior to the study. Study subjects were included after getting their informed consent.
3. Results
- Among 80 study subjects, 75 were females (94%). Most of the cases were from 31-50 age groups (81%) [Figure-1]. Obesity (84%), hypothyroidism (14%) and pregnancy (10%) were found as risk factors.
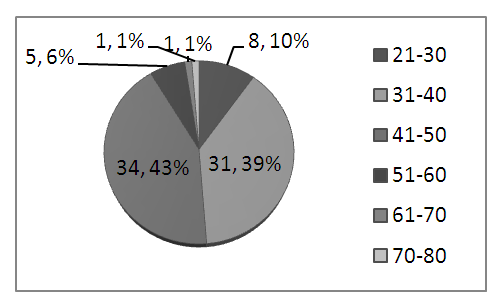 | Figure 1. Age distribution of study subjects |
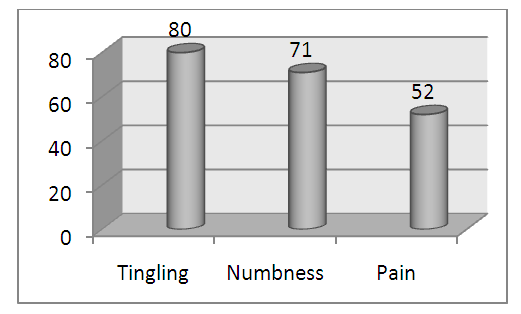 | Figure 2. Sensory symptoms in study subjects |
|
4. Discussion
- A strong female preponderance was observed in this study, similar to most other studies [12]. Obesity was found as the most prevalent associated factor as found by Alsharif et al [13]. Sensory symptoms assessed over 4 weeks, were reduced more in the intervention group than in the control group. This result is suggestive of the efficacy of Amitriptyline in reducing the sensory symptoms of early CTS.
5. Conclusions
- Amitriptyline is superior to placebo as a symptomatic treatment for the sensory symptoms of early CTS.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML