Ways Y. V.1, Yusupova S. M.1, Shakhmurova G. A.2, Khushbaktova Z. A.1, Syrov V. N.1
1Institute of Plant Chemistry Named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Tashkent State Pedagogical University Named after Nizami, Tashkent, Uzbekistan
Correspondence to: Shakhmurova G. A., Tashkent State Pedagogical University Named after Nizami, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Polyprenols, isolated from Alcea nudiflora and Vitis vinifera, when ones applied regularly to flat, full-thickness wounds on the skin of mice and rats, have a pronounced stimulating effect to the healing process. In this position, they are not inferior in activity, and in some cases, they are superior to well-known medicinal preparations with regenerative activity: sea buckthorn oil and 10% methyl uracil ointment.
Keywords:
Polyprenols from Alcea nudiflora, Polyprenols from Vitis vinifera, Wound healing action
Cite this paper: Ways Y. V., Yusupova S. M., Shakhmurova G. A., Khushbaktova Z. A., Syrov V. N., Influence of Polyprenols from Alcea Nudiflora and Vitis Vinifera to the Healing Process of Experimental Skin Wounds in Laboratory Animals, American Journal of Medicine and Medical Sciences, Vol. 11 No. 1, 2021, pp. 7-11. doi: 10.5923/j.ajmms.20211101.02.
1. Introduction
Polyprenols are one of the most frequently found biologically active substances in plants. They are unsaturated acyclic-branched spirits with a primary hydroxyl group in the terminal isoprene residue. The number and geometric configuration of these residues varies depending on the plant family [1]. Among the various biological activities of polyprenols [2,3,4,5], especially their ability stands out to accelerate regenerative processes in the body [6]. If we think that regeneration is one of the key of mechanisms for the restoration of the organism, both in the course of normal life, and when exposed to traumatic, chemical, thermal and other factors, threatening the viability of individual organs, systems and the organism as a whole, then a full-scale study of polyprenols in this regard will open up great possibilities for the creation of new effective preparations for use in the corresponding pathological conditions. This work presents the results of studying the wound-healing effect of polyprenols isolated from Alcea nudiflora and Vitis vinifera on various experimental animals.Purpose: To evaluate the study of the early healing effect of polyprenols isolated from plants Alcea nudiflora and Vitis vinifera in laboratory animals.
2. Materials and Methods
We used a outbred white mice - males (12-20 grams) and rats - males (190-200 grams) in the experiments. All animals were kept in stationary vivarium conditions on a normal food ration with free access to water. Experiments with them were carried out in accordance with the rules adopted by the International Convention for the protection of vertebrate animals, used for experimental purposes (Strsburg, 1986). Polyprenols isolated from the leaves of Alcea nudiflora L. [7] and from the leaves of Vitis vinifera subsp. Silvestris [8], conventionally named by us prenalon and vitaprenol, respectively. A pharmacological assessment of the effectiveness of polyprenols as agents, stimulating regenerative processes in the organism was carried out on models of destructive formations on the skin. In animals, at the site of the alleged application of the skin defect, the wool was previously cut, and the remnants of the wool cover were removed with 10% sodium sulfide (moistened for 2 - 3 minutes, followed by rinsing with warm running water).All traumatic procedures in animals were carried out under aseptic conditions under light ether anesthesia. Flat, full-thickness skin wounds in mice were reproduced in the dorsal region, using a special round stamp 9 mm in diameter, in rats – by excision of a skin flap with an area of 230 ± 1.2 mm2 to the fascia [9]. The observation of the healing process of experimental wounds was carried out until the complete recovery of skin defects. The state of the wound surface, the quality and maturity of granulations and the time of epithelialization were recorded. The area of wounds was measured according to Yupatov [10], the morphological assessment of their healing was carried out according to the description of Merkulov [11]. An integral quantitative assessment of the intensity of reparative processes was conducted by determining the value of the so-called reparative index, which takes into account in points the total severity of nine main morphological nonspecific signs of wound healing [12]. In some rats, Teflon rings were implanted into full-thickness circular skin defects on the back to prevent contraction and epithelialization. On the 7th day, the granulation tissue, developed in the defect (the moment of maximum development) was isolated, weighed and examined biochemically (deoxyribonucleic and ribonucleic acids, oxyproline, hexosamines, hexuronic acids, hexoses, sialic acids were determined [13,14]).The studied polyprenols (prenalon and vitaprenol), which are an oily liquid and reference preparations: sea buckthorn oil and 10% methyl uracil ointment (with high regenerative activity [15]), were applied to wound skin defects with a uniform layer once a day throughout the experiment. In parallel, there was a group of rats, the wound surface of which was not subjected to any treatment or was lubricated with sunflower oil (control). The obtained data during the experiments were processed by the method of variation statistics using t-criteria of the Student.
3. The Results of Research
The carried out experiments gave possibility to establish clearly that the regular application of the studied polyprenols to wound skin defects in mice and rats significantly accelerates their healing. Thus, in mice with clean planar skin wounds, from the first days of observation, the inflammatory reaction around wounds in animals, treated with prenalon and vitaprenol was less pronounced, and regenerative processes from the very beginning were more active than in control. They had less swelling, more meager wound exudate, active development of granulations. At the same time, in parallel with the development of connective tissue and vascularization of wounds, pronounced epithelialization of the wound defect was observed (the growing epithelium was clearly visible as a white rim against a dark red background of forming granulations). Most clearly, all of these processes took place on the wound surface of mice treated with vitaprenol. As a result, the healing of wound defects under its influence occurred on average 10 days earlier than in the control. Prenalon acted somewhat weaker, under its influence the wounds healed only for 7,8 days faster than controls.Prenalon and vitaprenol were superior in activity to sea buckthorn oil. In relation to methyl uracil ointment, their effect was different. Vitaprenol was significantly more active; prenalon had a similar effect (Table 1).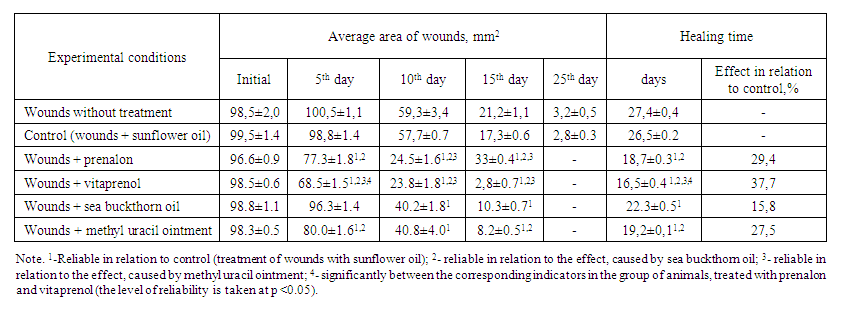 | Table 1. An influence of prenalon and vitaprenol in comparison with sea buckthorn oil and methyl uracil ointment on the healing of experimental skin wounds in mice (M ± m, n = 6) |
When prenalon and vitaprenol were applied to wound skin defects in rats, as well as in mice, a significant acceleration of their healing was noted, significantly superior to the effect of sea buckthorn oil. When it compared with methyl uracil ointment, the activating effect of prenalon on restoring the integrity of the skin, although it exceeded its effectiveness in some cases, these changes were not always reliable. Vitaprenol showed, as before, a more pronounced effect (Table 2).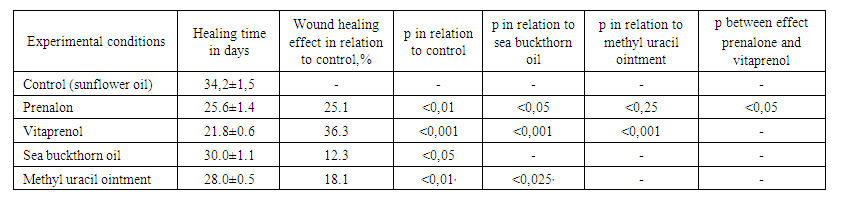 | Table 2. The influence of prenalon and vitaprenol in comparison with sea buckthorn oil and methyl uracil ointment on the timing of healing of full-thickness skin wounds in rats (M ± m, n = 6) |
Histomorphological analysis of the wound healing process showed that under the action of polyprenols, as well as reference-preparations (in the latter case, somewhat weaker), an earlier stimulation of the activity of cellular elements in the wound with an increase in the number of epithelial cells and fibroblasts is noted. The newly formed structures are characterized by an increased content of glycogen and ribonucleoproteins. An intensification of the growth of vascular elements, an acceleration of the formation of a demarcation shaft and rejection of a scab, a decrease in the severity of tissue edema, an increase in the rate of fibrillogenesis of collagen, maturation and transformation of granulation tissue, activation of epithelization are observed. This is especially clear, if we compare the total indices of nonspecific morphological signs of wound healing under the influence of polyprenols and comparison preparations (reparative indices) in control and experimental animals.As an example, Table 3 shows the obtained data in this plan after 14 days from the moment of reproduction of wounds. The presented materials reveal that the reparative index under the influence of prenalon and vitaprenol was 98.0 – 126.1%, and it was 60.8 – 111.8% higher than in the control under the influence of sea buckthorn oil and methyl uracil ointment.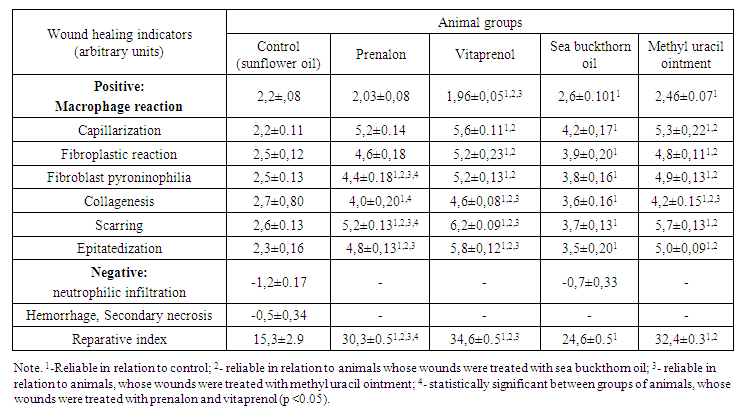 | Table 3. Indicators of the severity of reparative processes in planar full-thickness skin wounds of rats after their regular treatment with prenalon and vitaprenol in comparison with sea buckthorn oil and methyl uracil ointment on the 15th day of observation (M ± m, n = 6) |
Morphological (in particular, histochemical) analysis gives mainly an idea about structural and functional changes in cellular and fibrous elements in the process of regenerate formation [16]. However, in this case it was also important to characterize quantitatively this process by using biochemical methods. Therefore, under studying granulation tissue, formed in planar skin wounds of rats, treated with polyprenols, it was found that the content of deoxyribonucleic acids (cell count indicator) in it under the influence of prenalone and vitaprenol increased to 7.4 – 31.0%. In addition, ribonucleic acids involve (an indicator of the biosynthetic activity of cells) 48.8–61.0%. Under using the sea buckthorn oil and methyl uracil ointment, the content of deoxyribonucleic acids increased to 7.4 – 10.1, and ribonucleic acids – to 20.8 – 52.6%. These show about the ongoing stimulation of biosynthetic activity under the influence of polyprenols, and to a more pronounced degree than under the influence of reference preparations. An additional confirmation of this conclusion is an increase in the accumulation of collagen in the granulation tissue (judged by the definition of its marker - hydroxyproline [13]), which is an important component of connective tissue, and, which is also important, other connective tissue biopolymers – hexosamines, hexuronic acids and hexoses (Table 4). The glycoprotein components provide the optimization of conditions for collagen fibrillogenesis [17].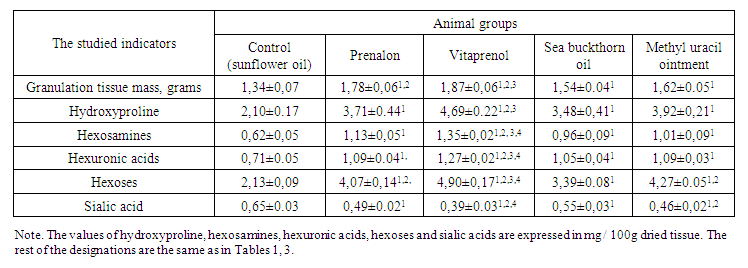 | Table 4. An influence of prenalon and vitaprenol in comparison with sea buckthorn oil and methyl uracil ointment on the development of granulation tissue in planar skin wounds of rats on the 7th day of observation and some indicators of its biochemical composition (M ± m, n = 6) |
The amount of granulation tissue itself on the 7th day of observation under the influence of prenalon, vitaprenol, and sea buckthorn oil and methyl uracil ointment was 32.8, 39.5; 14.9 and 20.9 more than in the control, respectively. From the same Table 4, it can be seen that the concentration of sialic acids in the granulation tissue of rats, treated with polyprenols and reference preparations decreased markedly, which may be a reflection of their inhibitory effect on inflammation in the regenerate area [13]. Under comparing of the obtained data, it can be seen that for all the parameters under consideration, the studied polyprenols (especially vitaprenol), as a rule, had a definite advantage over sea buckthorn oil and 10% methyl uracil ointment.So, according to the data of morphological and biochemical studies, polyprenols, isolated from Alcea nudiflora and Vitis vinifera, when they were applied to flat, full-thickness skin wounds of rats, have a pronounced stimulating effect on proliferative and biosynthetic processes in the cells of the epidermis and dermis. The influence to cells of these two main regenerative systems was manifested in the acceleration of the formation of epidermal regenerate and granulation tissue, which promoted faster wound healing. It should also be noted that under using polyprenols, conditions are created that promote full organotypic skin regeneration, since the wound healing process in this case proceeded (as opposed to control) without any deformation of the wound surface.The action of polyprenols from Alcea nudiflora – prenalon and Vitis vinifera – vitaprenol was generally similar to sea buckthorn oil and 10% methyl uracil ointment, but it manifested in a more pronounced degree (especially for vitaprenol), which is associated with their higher regenerative activity in the lesion.
4. Conclusions
The investigated polyprenols isolated from Alcea nudiflora and Vitis vinifera have a pronounced stimulation of regenerative processes and may have an early healing effect.
References
| [1] | Khidirova N.K, Shakhidoyatov Kh.M. 2002. Plant polyprenols and their biological activity // Chemistry of natural compounds, No. 2. – Pp. 87 – 98. |
| [2] | Syrov V.N., Khurshkainen T.V., Tsaruk A.V., Egamova F.R., Khushbaktova Z.A., Kuchin A.V. 2012. Adaptogenic properties of polyprenols, isolated from fir tree greens under immobilization stress // Chemical-Pharmaceutical Journal. – Vol. 46, No. 7. – Pp. 34 – 36. |
| [3] | Syrov V.N., Weiss E.V., Egamova F.R., Khushbaktova Z.A., Mamatkulova N.V., Shakhidoyatov H.M., Khidirova N.K. 2012. Antiulcer activity of polyprenols, isolated from cotton leaves // Chemical-Pharmaceutical Journal, Vol. 46, No. 3. – Pp. 34 – 36. |
| [4] | Vays E.V., Yusupova S.M., Khidirova N.K., Shakhmurova G.A., Syrov V.N. 2020. Experimental evaluation of the immunotropic effect of the polyprenols Vitis vinifera // European Science Review, No.5-6. – Pp. 22 – 26. |
| [5] | Vays E.V., Yusupova S.M., Khidirova N.K., Shakhmurova G.A., Egamova F. R. Khushbaktova Z.A. Zakirova U.T., Syrov V.N. 2020. Comparative study of hepatoprotective activity of the polyprenols from Vitis vinifera (Vitaprenol) and Carsil in experiences on rats with hepatitis, сaused by four-chloride carbon // Journal of Critical Review, Vol.7, No.13. – Pp. 724 – 727. |
| [6] | Roschin V.N, Sultanov V.S. 2005. Means for stimulating the processes of natural regeneration // Patent of the Russian Federation, No. 2252026, Bulletin of inventions, No.1. |
| [7] | Rakhmatova M.Zh. 2018. Neutral substances of plants Alcea rosea: Author’s abstract of the dissertation of the Doctor of Philosophy (PhD) in chemical sciences. – Fergana. – 49 p. |
| [8] | Zakirova U. T. Khidirova N.K., Tursunova N.V., Syrov V.N., Shakhidoyatov H.M. 2015. Polyprenols of Vitis vinifera leaves and their hepatoprotective activity // Chemistry of natural compounds, No.3. – Pp. 371 – 374. |
| [9] | Nikolayev A.V., Mamedov L.A., Berchenko G.N. 1977. Experimental - clinical aspects of reparative processes and methods of their stimulation. – Moscow. – Pp. 28 – 32. |
| [10] | Yupatov S.I., Lesko V.A. 1975. Treatment of patients with trophic ulcers // Surgery, No. 8. – Pp.52 – 55. |
| [11] | Merkulov G.A. 1969. Course of pathological and histological techniques. – Leningrad: Medicine. – 423 p. |
| [12] | Milovanova Z. P. Kantemirova B.F. 1981. Revealing the effectiveness of various collagen coatings on deep burn wounds by the method of morphological scoring // Experimental - clinical aspects of the use of biological polymers in medicine. – Moscow. – Pp. 26 – 28. |
| [13] | Slutsky L.I. 1969. Biochemistry of normal and pathologically altered connective tissue. – Leningrad: Medicine. – 375 p. |
| [14] | Trudolyubova M.G. 1977. Quantitative determination of ribonucleic and deoxyribonucleic acids in subcellular fractions of animal cells // Modern methods in biochemistry // Edited by V.N. Orekhovich. – Moscow: Medicine. – Pp. 313 – 316. |
| [15] | Mashkovsky M.D. 2008. Medicines. – Moscow: RIA “New Wave”. – Pp. 648 – 649; Pp.706 – 707. |
| [16] | Berchenko G.N., Ilyina T.M. 1977. Comparison of histochemical, ultra structural and biochemical data in the reparative histogenesis of connective tissue // Experimental - clinical aspects of reparative processes and methods of their stimulation. – Moscow. – Pp. 52 – 57. |
| [17] | Anderson J.C. 1976. Glycoproteins of the connective tissue matrix // Vol. 7. – P. 251. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
