-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(1): 1-6
doi:10.5923/j.ajmms.20211101.01
Received: Nov. 22, 2020; Accepted: Dec. 15, 2020; Published: Dec. 26, 2020

New Pathogenetic Mechanisms of Alopecia Areata
Azimova F. V., Sharakhmedov Sh. Sh.
Republic Specialized Scientific-Practical Medical Center of Dermatology and Venerology Ministry of Health of the Republic of Uzbekistan, Tashkent
Correspondence to: Azimova F. V., Republic Specialized Scientific-Practical Medical Center of Dermatology and Venerology Ministry of Health of the Republic of Uzbekistan, Tashkent.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The following studies are aimed at clarify the role of stem cells of the hair follicle that determine the rhythm of the latter during circular alopecia. Two important signal molecules, Bone Morphogenetic Protein (BMP) and Wingless-type (WNT), play a key role in the hair cycle during the telogen and anagen stages. We observed 54 patients with circular alopecia aged 18 to 46 years, of whom 12 were males and 42 females. The selection of patients and clinical part of the work was carried out on the basis of RSSPMCDV of MHRUz. The presented data indicate the leading role of BMP and Wnt signaling pathways in the pathogenesis of circular alopecia, which determines mitosis and differentiation of hair follicle cells, and open the perspectives of developing new effective drugs.
Keywords: Alopecia, Erythema, Dermal papilla, Hair follicle
Cite this paper: Azimova F. V., Sharakhmedov Sh. Sh., New Pathogenetic Mechanisms of Alopecia Areata, American Journal of Medicine and Medical Sciences, Vol. 11 No. 1, 2021, pp. 1-6. doi: 10.5923/j.ajmms.20211101.01.
- At present, thanks to fundamental research in cytogenetics and biochemistry, the morphology and physiology of the hair follicle has been significantly enhanced. The attention of researchers is drawn to the so-called bulge zone, a thickening located under the sebaceous gland, which some authors called the secondary hair germ. The biochemical and proliferative activity in the bulge zone even during the telogen phase (rest period) has been established and it has been proved that the hair growth cycle in the follicles occurs not only asynchronously, but also independently of the neighboring follicles, etc. [1,2,10,16,24]. During the whole hair cycle the repeated process of regress and regeneration can be observed only in the lower part of the follicle including the suprabulbar and bulbar areas. The internal regulation of the hair cycle is based on the interaction of two key populations of cells in the hair follicle - epithelial cells of the outer root vagina and mesenchymal cells of the dermal papilla and the dermal (connective tissue perifollicular) vagina. However, the location and type of inductors and anagen phase inhibitors have not yet been fully studied. At present, two important signal molecules - (Bone Morphogenetic Protein) (BMP) and (Wingless-type) WNT play a key role in the hair cycle during the telogen and anagen stage [3,5,15,29].Animal model studies have shown that activation of the Wnt-track is crucial to trigger follicle morphogenesis through activation of hair follicle stem cells. For stimulation of the anagen phase, there are always factors inhibiting the BMP signaling pathways [4,7,25] in addition to the activating Wnt-track signals. The increased activity of BMP-signals has been proved to preserve the hair follicle stem cells at rest during the period of telogen [8,11,13]. Scientists from different countries have carried out experimental studies that revealed that WNT signaling pathway is a key component in activation of Hair Follicle Stem Cells (HFSCs). HFSCs stimulate the regeneration of hair follicle cells, resulting in the growth of new hair. At the same time, the Hair Follicle Stem Cells (HFSCs) stay at rest for a few weeks before the signal for proliferation is received. Studies show that hair loss occurs when hair follicle stem cells (HFSCs) reach their end of life. HFSCs were found to be able to initiate WNT signals on their own, unlike most stem cells in other tissues [9,12,18,23]. The breakdown of the WNT Wnt signaling causes the hair follicle to delay in the telogen phase, which is manifested by the absence of hair growth. The researchers also found that the transmission of signals BMP (Bone Morphogenetic Protein - signal molecule of hair loss) can control the transmission of WNT signals. Uncontrolled transmission of WNT Wnt signals can stimulate premature activity of Hair Follicle Stem Cells (HFSCs), which in turn causes a premature transition to the anagen, which leads to disruption of hair chronobiology. The balanced interaction between the two signaling pathways makes it possible for HFSCs to stay at rest until the pre-programmed anagen stage occurs. Therefore, understanding the basic mechanisms of the hair growth cycle will open the way to discover new methods of hair growth recovery [17,20,26,27]. One of the most difficult-to-treat and recurring diseases is circular alopecia, which is currently considered a tissue specific autoimmune disease mediated by autoactivated T-lymphocytes in conditions of immune tolerance disorders by hair follicles in the anagenic stage. The conditions of manifestation, clinical diversity of circular alopecia are a complex combination of external and genetic factors [6,14,19,21,22,28].
1. The Aim of the Study
- Is to identify key molecules involved in initiation and suppression of the hair growth cycle in circular alopecia patients.
2. Study Materials and Methods
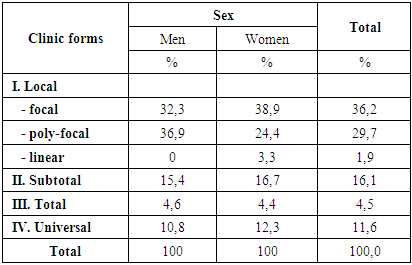
- We observed 54 circular alopecia patients aged 18 to 46 years, of whom 12 were males and 42 females. Patients were selected and the clinical part of the work was performed on the basis of RSSPMCDV of MHRUz. Inpatient records and outpatient records were used to study the clinical picture of alopecia, which took into account complaints, anamnestic data, general clinical trials and the results of special testing methods.As can be shown from Table 1, the main number of patients was with the local form of circular alopecia, among which there were 36.2% of patients with the focal form, 29.7% with the poly-focal form, and 1.9% with the linear form. Patients with subtotal form of circular alopecia were registered -16.1%. Patients with total form of diseases were 4.5%, universal form - 11.6%.
|
 | Picture 1. Total form |
 | Picture 2. Universal form |
|
|
3. Results and Discussion
- The cells of the dermal papilla isolated by us in the culture were proliferated, organized groups and even when the monolayer was reached, formed multilayer aggregates (so-called pseudopapillae). A number of functional features of the dermal papilla cells have also been revealed. There are indications in the literature that the hair follicle is the source of mesenchymal stem Cells (MSCs) that are involved in repairing the dermal papilla when it is damaged (Jahoda & Reynolds, 2001). We investigated the expression of dermal papilla surface markers, the BMP and Wnt proteins that carry the signal from the cell membrane to the nucleus. The deposition of lipid droplets in the cytoplasm and the expression of Leptin (a marker of mature adipocytes) were observed only in 20-30% of cells of the dermal papilla. Adipogenic differentiation of the dermal papilla cells was less expressed in patients with alopecia than in the control group. Spontaneous differentiation of the dermal papilla cells was not observed in patients with alopecia. The study results showed that patients with more severe forms of circular alopecia had positive BMP expression and negative Wnt1 expression. In these cases, morphogenetic protein signals transfer the hair follicle. Patients with mild forms of circular alopecia (focal, poly-focal) showed positive expression of Wnt1 and negative expression of BMP, proving the presence of mitosis and differentiation of the hair follicle cells, and lengthening of the hair follicle anagen stage. Undoubtedly, further studies based on the model will provide valuable data on the regulation of folliculogenesis induction in humans and prospects for finding agents that effectively regulate this process. (Pic. 3,4,5,6).
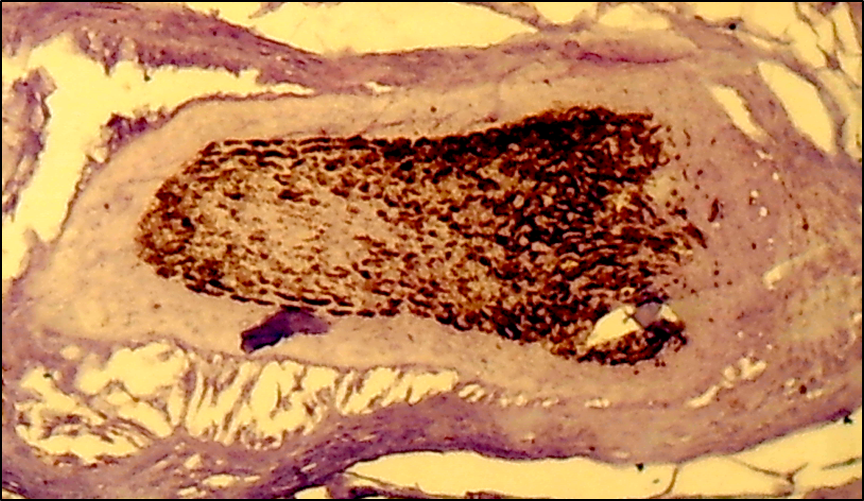 | Picture 3. Subtotal form of circular alopecia - hair follicle BMP-4: positive reaction of cells, cells are colored brown. V.40xСir. 20 |
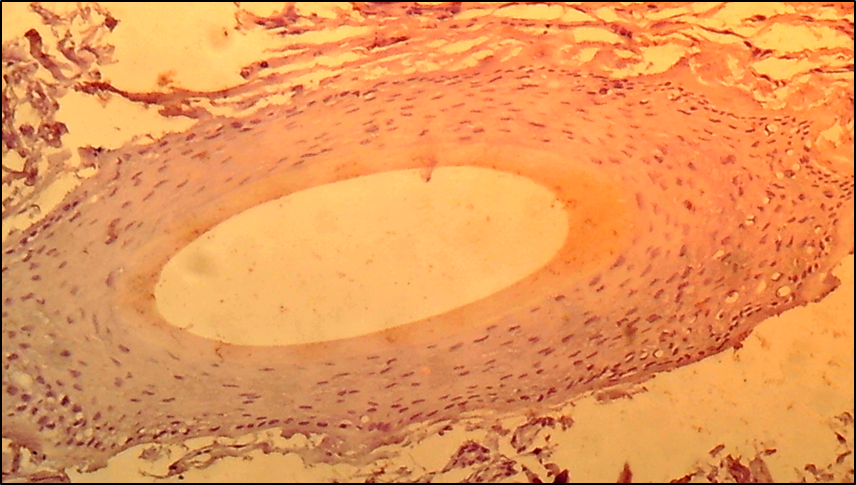 | Picture 4. Focal form of circle alopecia - hair follicle BMP-4: negative reaction of cells, cells are not colored. V. 40 x Cir. 20 |
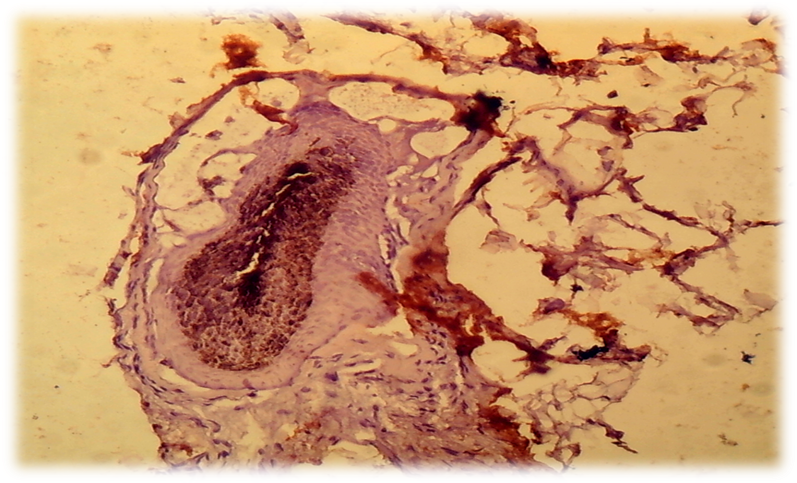 | Picture 5. Focal form of circle alopecia - hair follicle WNT1: positive reaction of hair follicles, cells are colored brown. V. 40 x Cir. 20 |
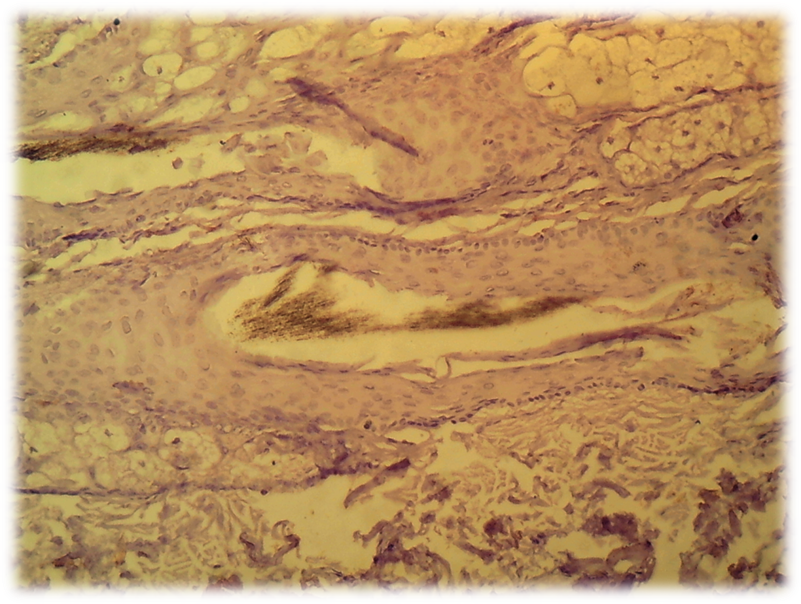 | Picture 6. Total form of circle alopecia - hair follicle WNT1: negative reaction of hair follicles, cells are not colored. V.40 x Cir. 20 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML