-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(12): 965-970
doi:10.5923/j.ajmms.20201012.08
Received: Nov. 11, 2020; Accepted: Nov. 28, 2020; Published: Nov. 30, 2020

Correction and Ways of Its Treatment with a High Risk of Thrombosis of the Vascular Access in Patients in Program Hemodialysis
B. T. Daminov, A. K. Sidikkhodzhaev
Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim is to study the effect of pentoxifylline on the pathogenetic aspects of vascular access thrombosis in patients requiring programmed hemodilysis. 187 patients with newly started hemodialysis were examined. All patients underwent an assessment of the risk of vascular access thrombosis according to the developed algorithm, as a result, 2 prognostic groups were formed: with a high risk of vascular access thrombosis (38 patients - 20.32%) and a low risk of access thrombosis (149 patients - 79.28%). The use of pentoxifylline during the year contributed to a significant (p <0.05 with baseline) decrease in the IR of the radial artery of the contralateral limb by 3.70%, while in patients in the low-risk group of diabetes mellitus thrombosis, an increase in IR by 1.55% was observed (p <0.05 with baseline data).
Keywords: Hemodialysis, Vascular access thrombosis, Duplex scanning of the vessels of the upper extremity, Endothelium-dependent vasodilation, Autoroset formation, Pentoxifylline
Cite this paper: B. T. Daminov, A. K. Sidikkhodzhaev, Correction and Ways of Its Treatment with a High Risk of Thrombosis of the Vascular Access in Patients in Program Hemodialysis, American Journal of Medicine and Medical Sciences, Vol. 10 No. 12, 2020, pp. 965-970. doi: 10.5923/j.ajmms.20201012.08.
1. Introduction
- Today, about 0.1-0.15% of the world's population requires programmed hemodialysis (HD) [1,2,3]. Hemodialysis can improve the survival and quality of life of patients with CKD [4]. Hemodialysis is possible using a catheter, arteriovenous shunt and arteriovenous fistula. AV shunt thrombosis occurs in 0.5-2% of patients per year, AV fistula - 0.1-0.5% per year. Thrombosis of the AV vascular access (DM) is associated with the interruption of the hemodialysis program, hospitalization of patients, and the installation of a temporary catheter. Diabetes thrombosis is the cause of 65-85% of diabetic dysfunction [5].In the aspect of diabetes mellitus and thrombus formation, Virchow's triad, including endothelial damage, blood stasis, and hypercoagulation, remains relevant [6]. DM endothelial damage is a complex problem. One component is a chronic abnormal increase in shear stress that causes endothelial dysfunction. Neointimal hyperplasia leads to disruption of the relationship between the endothelium and the excess connective tissue matrix on the basement membrane. Uremic toxins are also involved in the formation of endothelial dysfunction. Direct damage to the endothelium is caused by regular puncture of diabetes mellitus [7,8,]. Blood stasis in diabetes mellitus may be associated with stenosis of the efferent, efferent, or midsection (conduit). A small supply flow may be associated with low cardiac output, systemic hypotension, hypovolemia, and stenosis of the supply artery. In the area of the conduit, the localization of stenoses is predictable. In the case of an AV shunt, stenoses are observed proximal to the shunt-central vein anastomosis zone in more than 85% of cases [9], in the case of an AV fistula, it depends on the type of fistula. For example, in the case of a brachiocephalic fistula, the cephalic part is stenotic in 77% of cases; in a radiocephalic fistula, stenosis in 60% of cases is localized in the region of the superior anatomosing segment [10,11]. These stenoses result from a complex interaction of molecular and hemodynamic factors. Cytokines such as Hypoxia-Induced Factor-1alpha, Vascular Endothelial Growth Factor-A, Matrix Proteases and Platelet Growth Factor, produced by the endothelium under conditions of turbulent flow, abnormal shear stress, endothelial hypoxia, and trauma. These cytokines induce the migration of fibroblasts and vascular smooth muscle cells from the adventitia and media to the intima. Here they proliferate, leading to vascular stenosis [12]. Long compression times and overcompression after decanulation of diabetes can also cause stasis and thrombus formation.The role of hypercoagulability in diabetes thrombosis has long been underestimated. Uremia is associated with a violation of the functional state of platelets, which places HD patients in a cohort of persons with a hypercoagulable status [13]. Terminal CKD is a syndrome characterized by chronic activation of systemic inflammation, as evidenced by high levels of C-reactive protein. A high concentration of C-reactive protein is also one of the predictors of the risk of diabetes thrombosis [14]. Other risk factors for thrombosis associated with end-stage CKD include hyperhomocysteinemia, hypoalbuminemia, and hyperlipoproteinemia [15]. Blood circulation in the dialyzer and contact with the dialysis membrane also activates the coagulation cascade and platelet aggregation activity [16,17,18]. To correct these effects, heparinization of diabetes is used, but the systemic prothrombotic status remains. Hereditary thrombophilia also plays a role in the dysfunction of diabetes mellitus. In one study, at least 55% of patients with DM thrombosis were diagnosed with one or more prothrombotic syndromes [19]. The most common variants are Leiden factor V and prothrombin G20210A [20]. In patients with recurrent thrombosis, especially in the absence of significant stenosis, examination for hereditary thrombophilia is recommended [21].For the prevention of diabetes thrombosis, systemic antiplatelet agents and anticoagulants are used. In one meta-analysis, antiplatelet therapy was associated with a decrease in the risk of early thrombosis of AV fistulas (within the first month), but was ineffective in preventing long-term events, nor did it reduce the risk of thrombus formation and did not increase the duration of the so-called. "Mature" fistulas [22]. In a randomized trial, omega-3,6,9 polyunsaturated fatty acid preparations and dietary modification with the inclusion of seafood reduced the risk of AV shunt thrombosis by 50% [23]. In modern guidelines for the management of patients on HD, there are no recommendations regarding the use of anticoagulants.Objective: to study the effect of pentoxifylline on the pathogenetic aspects of vascular access thrombosis in patients requiring programmed hemodialysis.
2. Materials and Methods
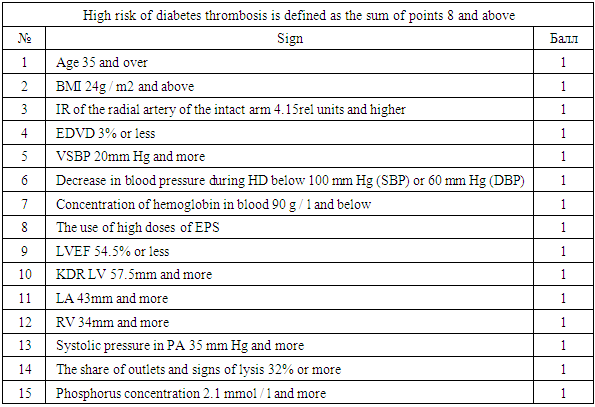
- The study included patients who received programmed hemodialysis at the Tashkent City Center for Nephrology and Kidney Transplantation. All patients underwent hemodialysis using diabetes mellitus in the form of an arteriovenous fistula (AVF) formed in the surgical department of this center. The time from the moment of surgery to the start of hemodialysis (the period of "maturation" of the fistula) ranged from 3 to 6 months.In our center, a risk scale for developing vascular access thrombosis was developed (Table 1).
|
|
3. Results and It’s Discussion
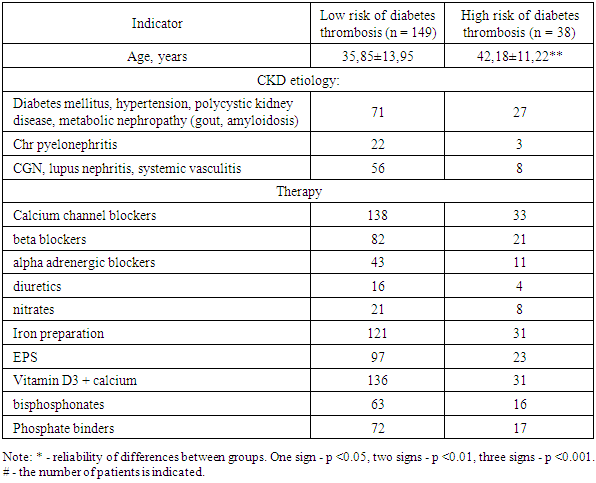
- The average age of the patients was 37.13 ± 13.66 years. Patients with a high score for the risk of thrombosis were, on average, older than those with a low score (p <0.01, Table 3).
|
4. Consclusions
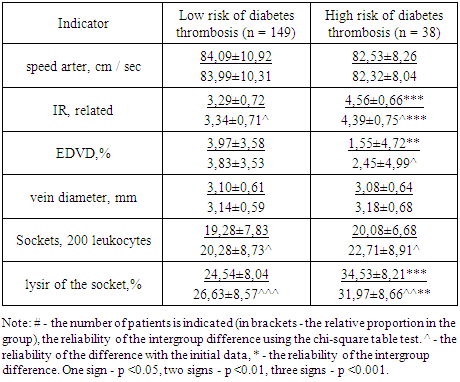
- Despite the multidirectional dynamics of the indicator, in the group of patients with a high risk of thrombosis of the vascular access, the proportion of outlets with signs of lysis at the end of the observation period remained significantly higher than in the group of patients with a low risk of thrombosis (p <0.01 reliability of intergroup differences). Long-term use of pentoxifylline at a dose of 600 mg / day in patients with a high risk of diabetic thrombosis contributes to a decrease in the IR of the radial artery, an increase in the degree of EDVD, and a decrease in the formation of lysed outlets. As a result, in the course of 12-month therapy with pentoxifylline, the score for the risk of diabetes thrombosis significantly decreased.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML