B. T. Daminov, A. K. Sidikkhodzhaev
Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Objective: to evaluate the preventive efficacy of pentoxifylline in the aspect of vascular access thrombosis in patients on programmed hemodialysis. 187 patients with first started hemodialysis were examined. All patients underwent an assessment of the risk of vascular access thrombosis according to the developed algorithm, as a result, 2 prognostic groups were formed: with a high risk of vascular access thrombosis (38 patients - 20.32%) and a low risk of access thrombosis (149 patients - 79.28%). During the follow-up, 27 patients developed vascular access thrombosis (14.44%). The distribution of patients who developed this complication, depending on the risk score, showed that 5 patients belonged to the group with a low risk of thrombosis (3.36%), 22 - to the high-risk group (57.89%, chi square = 71.11, p <0.001 reliability of the frequency difference between groups). When creating a scale for assessing the risk of developing vascular access thrombosis, we used a retrospective analysis of patients with diabetes mellitus thrombosis and uncomplicated diabetes mellitus.
Keywords:
Programmed hemodialysis, Vascular access thrombosis, Risk assessment scale, Echocardiography, Duplex scanning of the contralateral limb vessels, Autorotology, 24-hour blood pressure monitoring
Cite this paper: B. T. Daminov, A. K. Sidikkhodzhaev, Prevention of Vascular Access Thrombosis in Patients on Program Hemodialysis, American Journal of Medicine and Medical Sciences, Vol. 10 No. 12, 2020, pp. 956-961. doi: 10.5923/j.ajmms.20201012.06.
1. Introduction
In the modern world, due to progress in medical science and technology, as well as due to changes in lifestyle and epidemic situation, the number of patients in need of hemodialysis for the development of chronic kidney disease (CKD) is increasing. On average, up to 0.15% of the population need hemodialysis [1]. Various variants of vascular access are used for programmed hemodialysis - catheterization, AV fistula, AV shunt. The failure of the vascular access in 85% of cases is associated with thrombosis. According to the literature, vascular access thrombosis develops from 0.5 to 80% per year [2]. Thrombosis of the vascular access is associated with interruption of the hemodialysis program, increased cardiovascular risk, increased hospitalization and mortality rates, the need for temporary catheter placement and associated bacteremia, as well as with a significant increase in financial costs of treatment associated with the imposition of a new access, hemodialysis through temporary access, treatment of complications associated with dysfunction of vascular access and interruption of the hemodialysis program [3]. The developed methods of thrombus removal (endovascular and surgical) are not effective enough and in the overwhelming majority of cases it is necessary to form a new vascular access [4,5].AV shunt thrombosis occurs in 0.5-2% of patients per year, AV fistula - 0.1-0.5% per year [6]. Thrombosis of the AV vascular access (DM) is associated with the interruption of the hemodialysis program, hospitalization of patients, and the installation of a temporary catheter. Diabetes thrombosis is the cause of 65-85% of diabetes dysfunction [6]. In the case of thrombosis, in the absence of contraindications, endovascular thrombus removal is performed using pharmacomechanical or mechanical removal and lysis of the thrombus [7,8,9]. Given the increased frequency of CKD, including the terminal stage, requiring programmed hemodialysis, many studies formation of vascular access. Since 1997, the use of synthetic prostheses for the formation of diabetes decreases and the frequency of AV fistula formation increases.Objective: to evaluate the preventive efficacy of pentoxifylline in the aspect of vascular access thrombosis in patients on programmed hemodialysis.
2. Materials and Methods
The study included patients who received programmed hemodialysis at the Tashkent City Center for Nephrology and Kidney Transplantation. All patients underwent hemodialysis using diabetes mellitus in the form of an arteriovenous fistula (AVF) formed in the surgical department of this center. The time from the moment of surgery to the start of hemodialysis (the period of "maturation" of the fistula) ranged from 3 to 6 months.In our center, a risk scale for developing vascular access thrombosis was developed (Table 1).Table 1. Scale for the identification of HCKD patients with a high risk of diabetes thrombosis
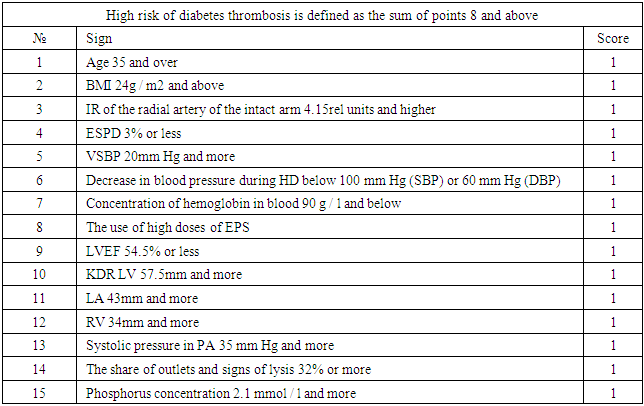 |
| |
|
187 patients with first started hemodialysis were examined. All patients underwent an assessment of the risk of vascular access thrombosis according to the developed algorithm, as a result, 2 prognostic groups were formed: with a high risk of vascular access thrombosis (38 patients - 20.32%) and a low risk of access thrombosis (149 patients - 79.28%). In high-risk patients, an antiplatelet agent (pentoxifylline 600 mg / day) was included in the therapy. The follow-up period was 1 year, by the end of which the incidence of the endpoint - DM thrombosis - was assessed in both prognostic groups. The clinical characteristics of the patients are presented in Table 2.Table 2. Clinical characteristics of patients included in the second stage of the study
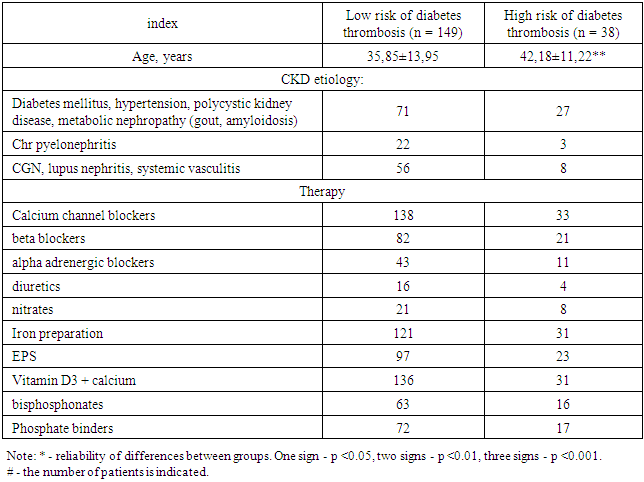 |
| |
|
Ultrasound examination of the vessels of the upper limb included duplex scanning (ultrasound scanning modes and spectral pulse-wave Doppler mode) of the arteries and veins of the contralateral (without DM) arm. The study was carried out in the morning hours (8-11 am) on the second day after hemodialysis, in the supine position after 10 minutes of rest, using an ultrasound scanner equipped with a linear transducer with a frequency of 7.5 MHz. The following indicators were recorded:- maximum systolic blood flow velocity in the radial artery;- the final diastolic blood flow velocity in the radial artery;- the resistivity index (Pursello index, IR) was calculated: IR = (systolic velocity-diastolic velocity) / systolic velocity;- the diameter of the brachial artery is 2-5 cm above the elbow bend;- a test with endothelium-dependent vasodilation (EDVD) of the brachial artery was performed, for which a tonometer cuff was applied to the shoulder, the pressure in which was pumped 50 mm Hg higher than the previously measured systolic blood pressure (SBP). The compression lasted 5 minutes, after which, after the release of compression, at 60 seconds, the diameter of the brachial artery was re-measured and the EZVD was calculated: EZVD = (diameter of the brachial artery after release of compression-diameter of the brachial artery initially) / diameter of the brachial artery initially * 100.Echocardiography. To assess the characteristics of central hemodynamics, all patients included in the study underwent echocardiography (EchoCG) using an ultrasound scanner equipped with a sector transducer with a frequency of 4.2 MHz. The study was carried out on the 2nd day after hemodialysis, in the morning hours, in the position of lying on the left side and lying on the back, with the left hand located under the patient's head. During echocardiography, standard echocardiographic positions and projections were used. To determine the measurement time, the synchronization of the image with the electrocardiogram was used. The following indicators were recorded:In the parasternal position, projection along the long axis of the left ventricle- end diastolic size of the left ventricle (LV EDD);- the final systolic size of the LV;- anteroposterior diameter of the left atrium (LA);- the diameter of the right ventricle (RV).LV end-systolic and diastolic volumes (Teichholtz method) and LV ejection fraction (EF) were calculated. LVEF = (LV end-diastolic volume-LV end-systolic volume) / LV end-diastolic volume * 100.In the case of a change in the geometric shape of the LV (spherical deformation of the cavity, the presence of an aneurysm or regional dyskinesis), vertical position of the heart or LV angulation, or LV EDD exceeding 54 mm, LV volumes for calculating LVEF were measured by the Simpson method using apical 2 and 4- x chamber echocardiographic position.- systolic pressure in the pulmonary artery (PA systolic) was calculated using tricuspid regurgitation, which was recorded in the parasternal position along the short axis at the level of the aortic valve with focus on the tricuspid valve or in the apical 4-chamber position (the maximum recorded velocity was used). After mapping tricuspid regurgitation by color pulse-wave Doppler sonography, registration of the peak velocity of tricuspid regurgitation was performed by non-first-wave Doppler sonography. The Bernoulli equation (pressure gradient = 4 * peak velocity 2) was used to calculate the peak systolic pressure gradient across the tricuspid valve. SystR PA was defined as the sum of the peak systolic pressure gradient across the tricuspid valve and the pressure in the right atrium. The pressure in the right atrium was determined by a semi-quantitative method using ultrasound scanning of the inferior vena cava with determination of its diameter during calm breathing and its collapse during deep inspiration (subcostal position, projection of the inferior vena cava), as well as measuring the diameter of the right atrium (apical 4-chamber position) (45). 24-hour blood pressure monitoring (ABPM) was carried out on the second day after hemodialysis using the BTL ABPM complex (UK). ABPM lasted for 24 hours, BP was measured every 30 minutes during the day and every 60 minutes at night. The measurement method is oscillometric. During the study, the following calculated data were used:- the average daily level of systolic blood pressure (SBP) - the arithmetic mean of all registered indicators of systolic blood pressure;- the average daily level of diastolic blood pressure (DBP) - the arithmetic mean of all registered indicators of diastolic blood pressure;- daily index (SI) = (mean daytime hemodynamic blood pressure-mean nighttime hemodynamic blood pressure) / mean hemodynamic daytime blood pressure * 100, where mean hemodynamic blood pressure was the arithmetic mean of all hemodynamic blood pressure indicators for the corresponding period of time. Hemodynamic blood pressure = (SBP-DBP) / 3;- variability of SBP (VSAP) - standard deviation of all SBP indicators recorded during the day;- DBP variability (SDPP) - standard deviation of all DBP indicators recorded during the day.In addition, in the course of the study, blood pressure was monitored during the hemodialysis procedure and episodes of arterial hypotension were recorded - a decrease in SBP below 100 mm Hg. or DBP below 60 mm Hg. Blood pressure was measured by the Korotkov method using a Riva-Rocchi tonometer every 20 minutes.Laboratory research.During the study, all patients underwent a general blood test using an automatic analyzer. The concentration of hemoglobin in the peripheral venous blood was analyzed. Blood sampling was carried out on the 2nd day after the hemodialysis procedure, on an empty stomach at 7-8 in the morning, after 10 minutes of rest, in a sitting or lying position. The cubitalipsilateral vein was used. Blood sampling was carried out in vacutainers containing an anticoagulant.To study the state of mineral metabolism, the concentration of parathyroid hormone and phosphorus in serum was determined during the study. Venous blood was taken from the cubital vein of the ipsilateral arm, on an empty stomach, at 7-8 am after 10 minutes of rest, in a sitting or lying position, on the 2nd day after hemodialysis. Determination of the phosphorus concentration was carried out by the biochemical method, parathyroid hormone - by the enzyme immunoassay.To determine the aggregation and rheological properties of blood in the course of the study, the phenomenon of auto-socket formation was studied. For this purpose, on the 2nd day after hemodialysis, on an empty stomach, in a lying or sitting position, in the morning hours (7-8 hours), after a 20-minute rest, capillary blood was taken. In a smear of native blood, the number of outlets was counted - an accumulation of cellular elements of blood, in the center of which there is a rosette-forming cell - a leukocyte, to which erythrocytes and platelets are attached. The number of outlets per 200 leukocytes was counted. In addition, among the outlets, the proportion of lysed outlets was recorded with signs of damage to the cell membrane and disruption of the integrity of the cells forming the outlet.All received data were entered into Excell summary tables. After the formation of groups, all parameters were described in the form of the arithmetic mean and its standard deviation. The significance of intergroup differences was determined using the Student's t test. Comparison of the frequency of occurrence of signs between groups was carried out using the tabular chi-square test and checking its reliability against the tables depending on the number of degrees of freedom.
3. Results and It’s Discussion
To assess the prognostic efficacy of the developed diabetes thrombosis risk scale, 187 patients were examined, for whom an AV fistula was first installed for program HD. The patients were examined according to the proposed protocol. The average score calculated on the proposed scale was 6.11 ± 2.44 points. The most common score was 6 (44 patients) and 5 (41 patients) points (Fig. 1). Thus, a group of patients with a high risk of diabetes thrombosis was identified, defined as a score of 8 or more (38 patients - 20.32% 0).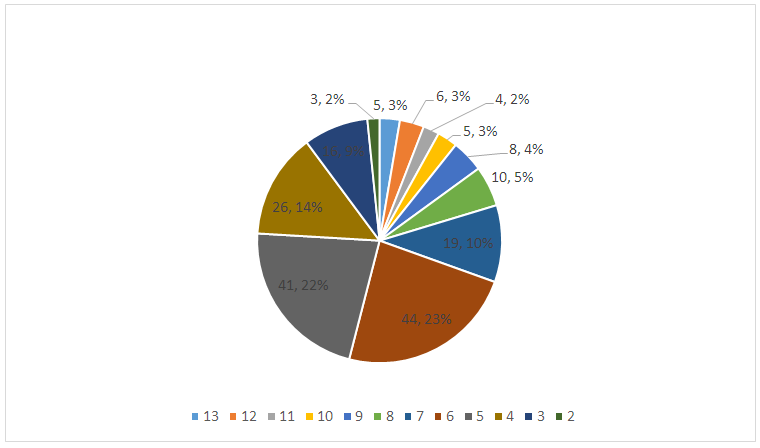 | Figure 1. Distribution of HCKD patients according to the score for the risk of diabetes thrombosis |
The average age of the patients was 37.13 ± 13.66 years. To study the etiological characteristics of patients included in the study, all causes of HCKD were divided into two groups: one included patients with primary inflammatory kidney damage - glomerulonephritis, pyelonephritis, nephritis in connective tissue diseases, systemic vasculitis. There were 89 such patients (47.59%). The second group of patients consisted of patients with vascular, metabolic diseases and impaired renal development (diabetes mellitus, arterial hypertension, polycystic kidney disease, gouty nephropathy) - 98 patients (52.41%). Among patients with a high risk of diabetes thrombosis, patients of the second group (71.05%) prevailed, among patients with a low risk - inflammatory diseases (52.35%, chi square = 6.71, p <0.01, Fig. 2).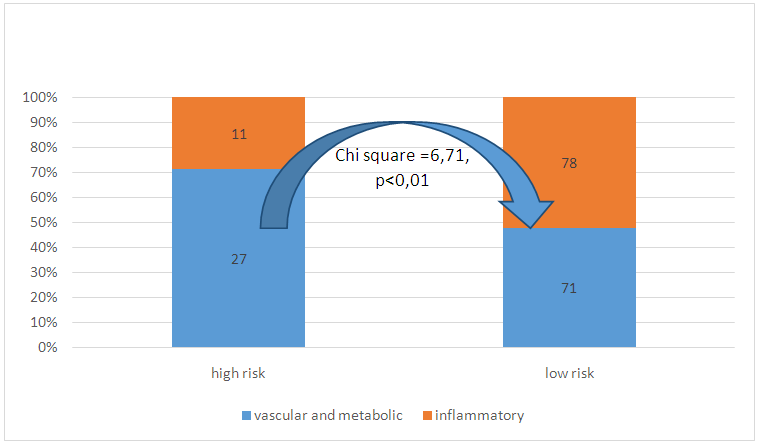 | Figure 2. Distribution of patients with high and low risk of thrombosis depending on the etiological group |
All patients allocated to the high-risk group were prescribed pentoxifylline in a daily dose of 600 mg. The drug combines the properties of an antiplatelet agent and a venotonic agent.During the follow-up, 27 patients developed vascular access thrombosis (14.44%). The distribution of patients who developed this complication, depending on the risk score, showed that 5 patients belonged to the group with a low risk of thrombosis (3.36%), 22 - to the high-risk group (57.89%, chi square = 71.11, p <0.001 reliability of the frequency difference between groups). When creating a scale for assessing the risk of developing vascular access thrombosis, we used a retrospective analysis of patients with diabetes mellitus thrombosis and uncomplicated diabetes mellitus. Patients with a score of 8 points (31 patients) and higher thrombosis of the vascular access developed in 29 patients (93.55%), while among patients with a score below (29 patients), only 1 person (3, 45%, chi square = 48.72, p <0.001). The relative risk of diabetes thrombosis in patients with a score of 8 or more is 27.13 times higher than among patients with a total score below 8. Comparison of the incidence of diabetes thrombosis showed that among patients with a score of less than 8 points for the risk of vascular access thrombosis thrombosis of the vascular access was comparable (Fig. 3): 3.45% in the first branch of the study and 3.36% in the second branch of the study (chi square = 0.30, nd), while among patients with a high score (8 or more points), significant differences were found: during the first branch of the study, thrombosis was detected in 93.55% of patients in the high-risk group, and in the second branch, during which pentoxifylline was used in this group of patients at a dose of 600 mg per day, thrombosis developed in 57.89% of patients (chi square = 11.42, p <0.001). Thus, the use of pentoxifylline at a dose of 600 mg / day for a year can reduce the risk of vascular access thrombosis in patients with a high risk by 1.62 times (or 38%).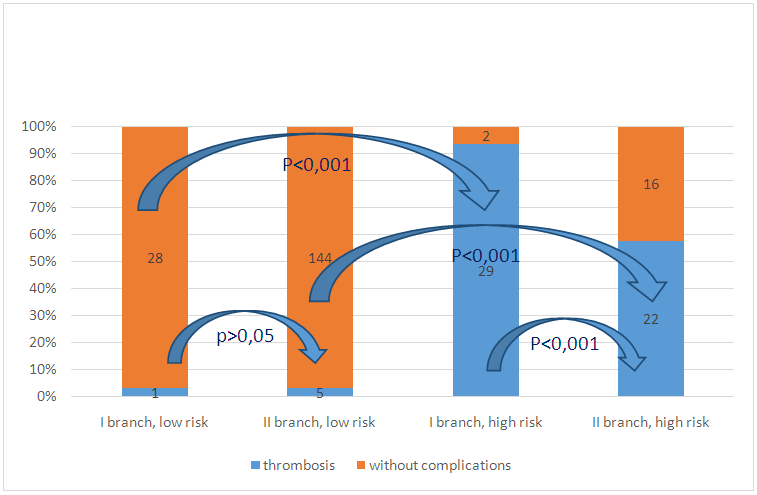 | Figure 3. Frequency of diabetes thrombosis in patients included in the study, depending on the risk score and the branch of the study |
Thus, the present study has demonstrated that the distribution of CKD patients receiving programmed hemodialysis for less than 1 year according to the proposed risk assessment of vascular access thrombosis makes it possible to identify patients at high risk - those who have more than 8 predictive markers of complications. The use of pentoxifylline in this group of patients can effectively reduce the risk of thrombosis.
4. Consclusions
The use of the methods developed in the course of this study: the isolation of patients with a high risk of diabetes thrombosis using the developed method and long-term (12 months) use of pentoxifylline in this group of patients at a dose of 600 mg / day reduces the risk of developing vascular access thrombosis by 38%.
References
| [1] | Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017; 69: A7-8. |
| [2] | Vascular Access Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48 Suppl 1: S248-73. |
| [3] | Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol. 2013; 9: 348–357. |
| [4] | Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am SocNephrol. 2011; 6: 1996–2002. |
| [5] | Rayner HC, Besarab A, Brown WW, et al. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines. Am J Kidney Dis. 2004; 44 (suppl 2): 22–26. |
| [6] | Quencer KB, Friedman T. Declotting the Thrombosed Access. Tech VascIntervRadiol 2017; 20: 38-47. |
| [7] | Roberts AC, Valji K, Bookstein JJ, et al. Pulse-spray pharmacomechanical thrombolysis for treatment of thrombosed dialysis access grafts. Am J Surg 1993; 166: 221-5; discussion 225-6. |
| [8] | Trerotola SO, Vesely TM, Lund GB, et al. Treatment of thrombosed hemodialysis access grafts: Arrow-Trerotola percutaneous thrombolytic device versus pulse-spray thrombolysis. Arrow-Trerotola Percutaneous Thrombolytic Device Clinical Trial. Radiology 1998; 206: 403-14. |
| [9] | Cynamon J, Lakritz PS, Wahl SI, et al. Hemodialysis graft declotting: description of the "lyse and wait" technique. J VascInterv Radiol 1997; 8: 825-9. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
