-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(12): 940-945
doi:10.5923/j.ajmms.20201012.03
Received: Nov. 3, 2020; Accepted: Nov. 26, 2020; Published: Nov. 30, 2020

Antigen-Binding Lymphocytes in Patients with Hyperplastic Endometrial Processes to Tissue Membrane Antigen with Uterine Myomas
Nazirova Zilola
Department of 1st Obstetrics and Gynecology Faculty, Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Nazirova Zilola, Department of 1st Obstetrics and Gynecology Faculty, Andijan State Medical Institute, Andijan, Uzbekistan.
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

HPE is a hyperplastic process of the endometrium. This is an oncological pathology, but it is considered a benign formation that affects the mucous layer of the uterus. Among gynecological pathologies, HPE in gynecology is characterized by a thickening of the endometrium, which envelops the walls of the uterus. As a result, the cells of the mucous membrane grow in an unusual way for themselves, which entails an increase in the uterus.
Keywords: Myoma, Adenomyosis, Hyperplasias, Endometritis, Submucous, Echographic, Biological, Hyperplasia
Cite this paper: Nazirova Zilola, Antigen-Binding Lymphocytes in Patients with Hyperplastic Endometrial Processes to Tissue Membrane Antigen with Uterine Myomas, American Journal of Medicine and Medical Sciences, Vol. 10 No. 12, 2020, pp. 940-945. doi: 10.5923/j.ajmms.20201012.03.
1. Introduction
- Endometrial hyperplastic processes (HPE) are among the most common gynecological diseases, occurring with a frequency of 30 to 55% [6,10].The important clinical significance of this pathology lies in the fact that HPEE is one of the most common causes of uterine bleeding and hospitalization of women. Many authors note the high frequency of the combination of HPE with uterine myoma, adenomyosis, which are often preceded by chronic inflammatory processes of the endometrium [2].The likelihood of HPEE malignancy continues to be the subject of discussion and research for more than a decade [4,9]. Determining the possibility of malignancy of the hyperplastic endometrium is complicated by the fact that, despite the pronounced clinical symptoms of this disease, some patients do not have uterine bleeding and they remain underexamined and do not receive timely treatment. Another problem is the difficulty of differential diagnosis between atypical hyperplasia and highly differentiated adenocarcinoma.According to the literature, only 2% of endometrial hyperplasia without atypia is transformed into adenocarcinoma, and 25% of atypical hyperplasias become malignant [12]. However, HPE is referred to as proliferative conditions and, in this regard, is considered in the oncological aspect.Currently, the study of the molecular mechanisms of the development of various pathological conditions continues. Understanding the basic principles of cell growth induction, especially in conditions of tumor transformation of cells, is an integral part of a competent approach to the management and monitoring of proliferative activity [6].Molecular biological mechanisms of the development of HPE are actively studied. We must agree with the opinion of researchers who believe that HPE is a polyetiological disease - the onset and progression of which is facilitated by a variety of factors and causes [1,5].The role of unbalanced estrogenic stimulation in the development of HPE is well known. High concentrations of estrogens stimulate the proliferative potential of cells, regulate the processes of angiogenesis, and reduce the ability of cells to repair DNA and antitumor in HPE unit [8,12]. Thus, in pathological conditions accompanied by hyperestrogenism, favorable conditions and prerequisites are created for tumor transformation of the endometrium. Estrogens can stimulate the division of endometrial cells that have already had any mutations in their DNA, or have acquired them.However, there are research data on the possibility of developing HPE against the background of the absence of hormonal disorders, which indicates the presence of other mechanisms for the formation of endometrial hyperplasia [3].In addition to hormones, cytokines, growth factors, proliferation markers, components of the extracellular matrix are activators of the proliferative activity of the endometrium in normal and pathological conditions [7,9,10]. Research is continuing on the processes of apoptosis in the genesis of proliferative diseases. A new direction - the study of the pathogenesis of the combination of HPE and another important gynecological pathology - chronic endometritis - is of great relevance and importance.Issues related to the treatment of HPE are still very relevant due to the high recurrence rate of this disease. Today in the arsenal of modern medicine there are various approaches to the treatment of HPE. Hormone therapy (combined oral contraceptives, gestagens, antiprogestins, gonadoliberin agonists) remains one of the most common methods of treating PEE without atypia. However, according to a number of studies, the effectiveness of hormonal methods of treating HPE without atypia is low - 42% [4]. In addition, conservative treatment is not differentiated and does not take into account the presence of combined endometrial pathology.The study of the morphological features of the combination of HPE and chronic endometritis, the severity of proliferative activity, the characteristics of the nature of their vascularization and receptivity allows us to reveal some new pathogenetic mechanisms in combined pathology of the uterus. This is especially important for prevention and effective therapy.Thus, the high incidence of HPE, the lack of proper efficacy from hormonal therapy, as well as the likelihood of their malignancy, puts the problem of the development and pathogenetic treatment of HPE among the most important of modern medicine.
2. Materials Methods
- To achieve this goal and objectives, a prospective study was carried out, which included 1490 patients who were hospitalized from 2004 to 2011 in gynecological departments.The indications for hospitalization of women in the hospital were: uterine bleeding of various nature (85.6%) and/or the presence of echographic signs of pathological changes in the endometrium (14.4%). Criteria for the inclusion of patients in the study: peri-menopausal period, absence of cancer; absence of endocrine pathology (diabetes mellitus, hypo- and hyperthyroidism, obesity P-III st); the absence of an acute inflammatory process of the pelvic organs; informed voluntary consent of the patients to carry out all the necessary medical and diagnostic procedures.The study did not include patients with endometrial polyps, large uterine myoma, and submucous localization of myomatous nodes, adenomyosis II-III stage. After the examination, which included clinical, laboratory, and echographic methods, all patients underwent separate diagnostic curettage of the uterus under the control of hysteroscopy. Depending on the results of the histological examination of scrapings from the cervical canal and uterine cavity at the first stage of the study, women were divided into two groups. The comparison group consisted of 870 patients in whom, according to the morphological study, no pathological changes in the endometrium were revealed, and the main group, which consisted of 620 women with the histologically confirmed hyperplastic endometrial process.
3. Results and Discussions
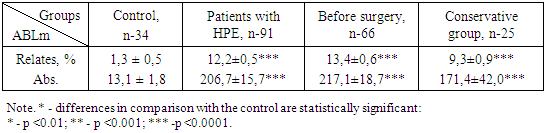
- It was revealed that in the blood of healthy women, the content of ABLm that responds to tissue antigen HPE is 1.3 ± 0.3% with individual fluctuations from 0 to 3%. In patients with HPE, the content of ABLm in blood serum averaged 12.2 ± 0.5% (p <0.001), with the limits of individual fluctuations from 2 to 27%.The normative values were calculated within M ± 2b, the upper limit of which was 2.9%, in connection with which the test for the presence of HPE was considered positive in women whose blood ABLm level exceeded 4%.We did not find a significant relationship between the relative and absolute indices of ABLm on the size of the tumor (Fig. 1).
 | Figure 1. ABLm indices in the peripheral blood of women with HPE depending on the size of the "uterus-tumor" complex |
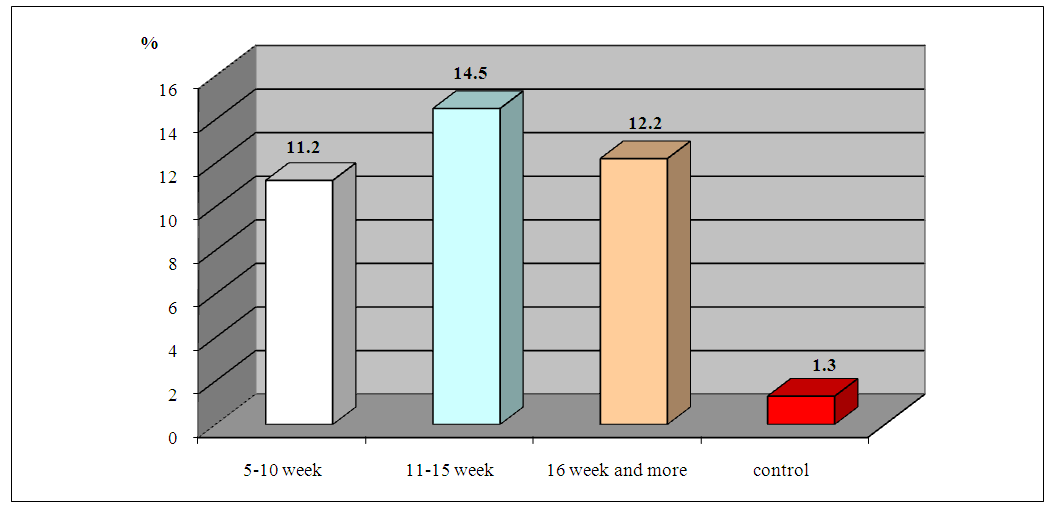
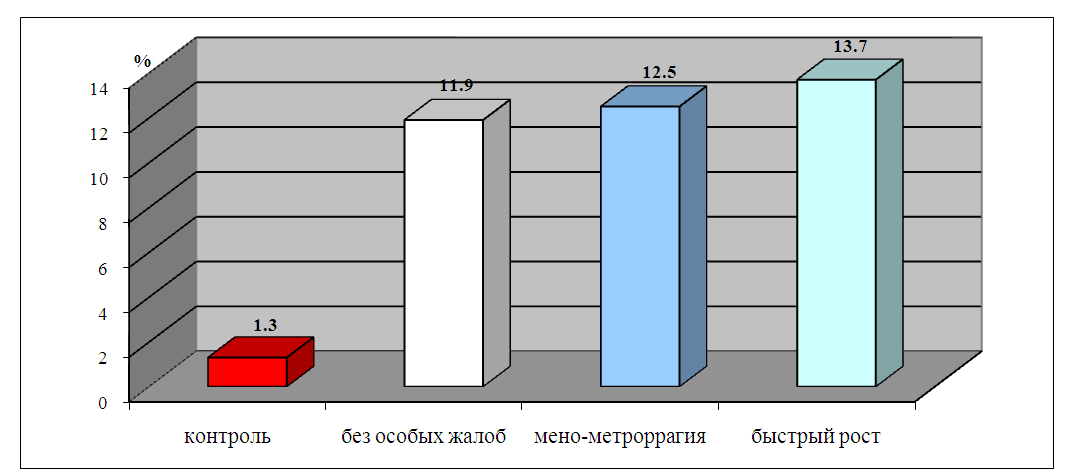 | Figure 2. ABLm indices in the peripheral blood of women with HPE depending on the clinical features of the disease |
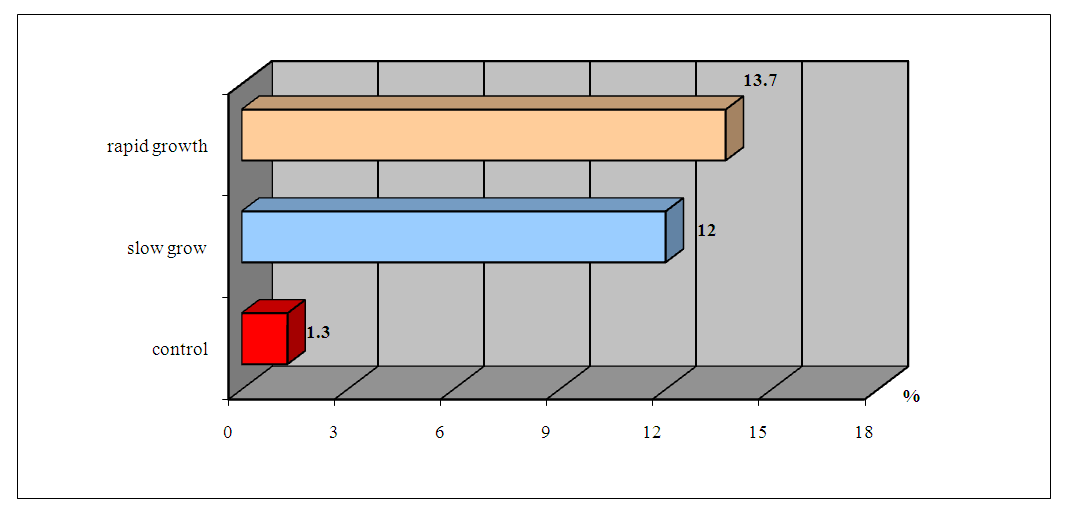 | Figure 3. ABLm indices in the peripheral blood of patients with HPE depending on the rate of tumor growth |
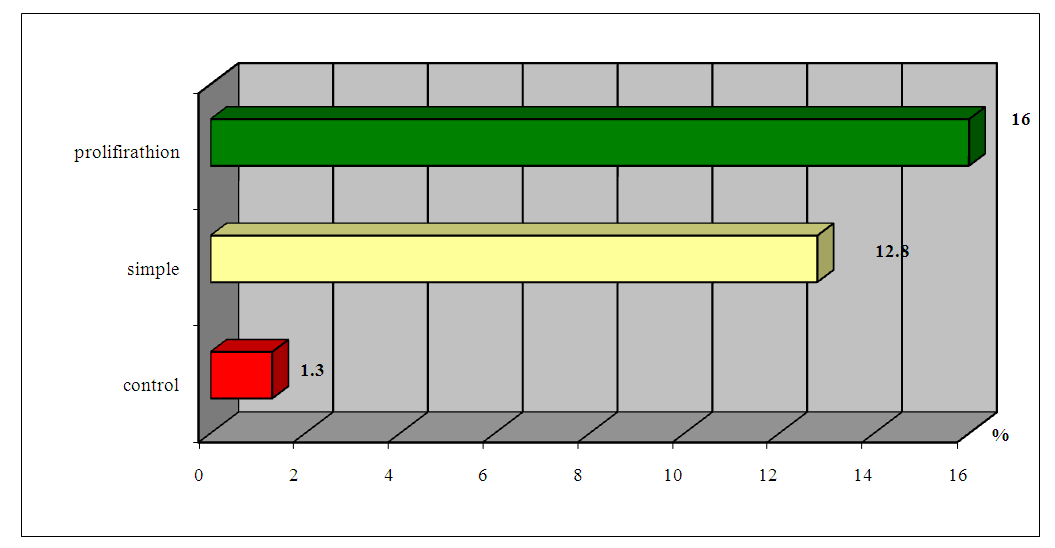 | Figure 4. Indicators of ABLm in peripheral blood depending on the morphogenetic type of HPE |
|
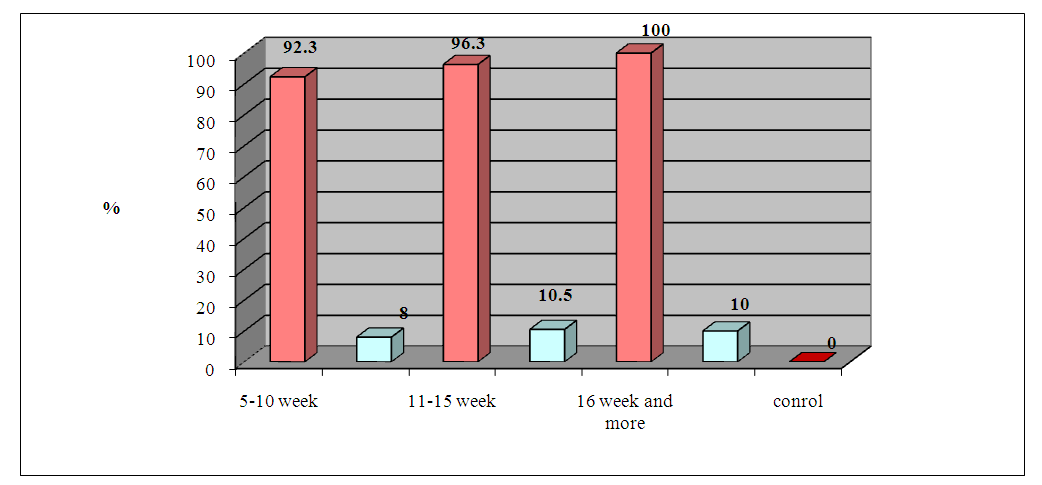 | Figure 5. Frequency of positive ABLm tests depending on the size of the tumor before and 1 month after radical treatment |
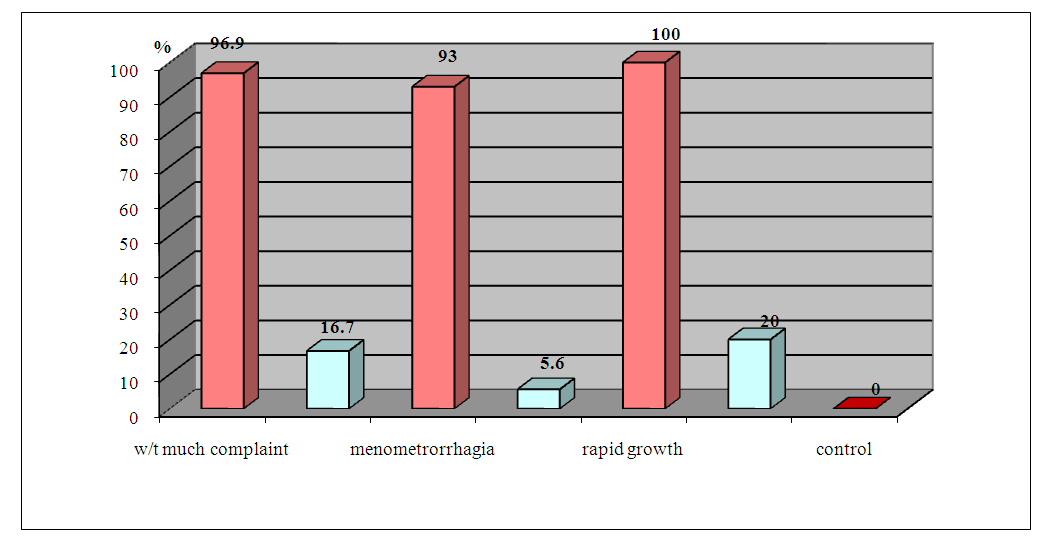 | Figure 6. Frequency of positive samples ABLm depending on the clinical course of GGE before and 1 month after radical treatment |
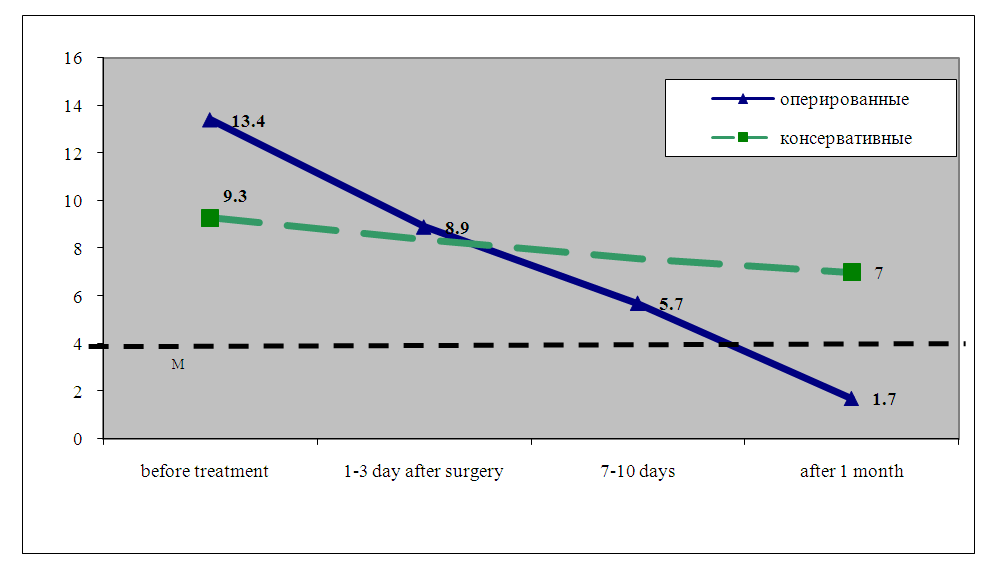 | Figure 7. Dynamics of ABL after surgical and conservative treatment of HPE |
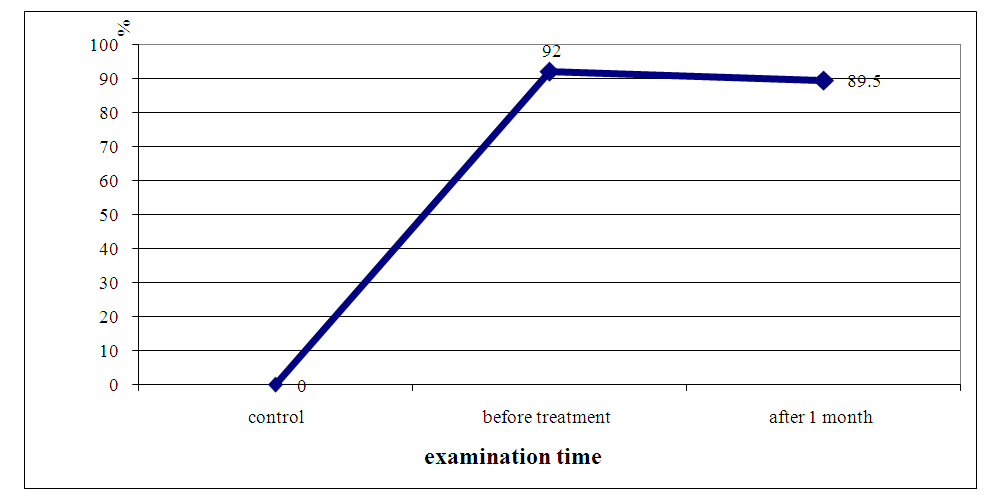 | Figure 8. The frequency (in%) of detection of ABLm in unoperated patients with HPE before and 1 month after the therapy with HPE immune modulator |
4. Conclusions
- Studies have shown that women with HPE have a sharp increase in the number of ABLm. Consequently, the reaction of determining the total pool of antigen-binding T and B cells in patients with HPE is specific and can serve as a criterion for the presence of HPE and assessing the effectiveness of the treatment. In our opinion, the high level of ABLm also confirms the position of the presence of an autoimmune mechanism acting as a factor in enhancing tumor growth.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML