-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(12): 934-939
doi:10.5923/j.ajmms.20201012.02
Received: Nov. 2, 2020; Accepted: Nov. 25, 2020; Published: Nov. 30, 2020

Treating Iatrogenic Pseudoaneurysms with an Injection of Thrombin Using a US-Guided
Khomidov Feruz
Department of Medicine Graduate School, Tashkent Medical Academy, Uzbekistan
Correspondence to: Khomidov Feruz, Department of Medicine Graduate School, Tashkent Medical Academy, Uzbekistan.
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

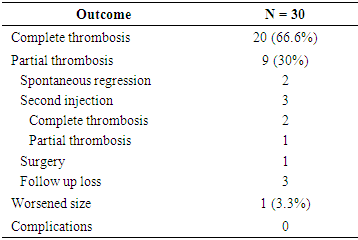
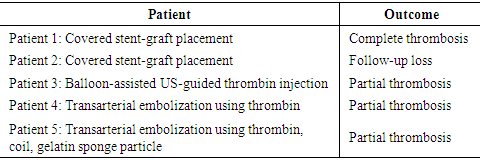
The treatment of aortoiliac occlusive disease has shifted to endovascular therapy. Even in complex lesion, precise angiography enables successful treatment in endovascular work. 37 patients with post-procedure femoral artery pseudoaneurysm were managed from March 2009 and June 2019. The lesion was confirmed by US or CTA. The characteristics of the lesion were analyzed. The pseudoneurysms were treated by US guided thrombin injection or endovascular technique. The sex ratio (male: female) was 24: 13. The mean age was 67.89 years. The mean diameter was 22.4 mm (5 - 45 mm). The etiology was as follows: PTA of lower extremity (40%), cardiac intervention (32%), transarterial embolization of internal organ bleeding (16.2%), and CRRT application (10%). Thirty-two patients were treated with percutaneous US-guided thrombin injection, and the median dose of thrombin injected was 453 U (range = 100–1300 U). Complete sac exclusion in 20 (66.6%) patients and partial exclusion in 9 (30%) patients were obtained. One lesion was increased in size and eventually had surgery. Five patients were treated with endovascular technique as follows: covered stent deployment (2), balloon-assisted US-guided percutaneous thrombin injection (1), coil embolization (1), thrombin injection (1). The complete sac exclusion was shown in one patient who had covered stent deployment, and partial exclusion in remaining 4 patients. Percutaneous management of femoral artery pseudoaneurysm can be the fast and effective tool.
Keywords: Pseudoaneurysm, Transarterial, Endoluminal, Sonographic, Thromboplastin, Clopidogrel
Cite this paper: Khomidov Feruz, Treating Iatrogenic Pseudoaneurysms with an Injection of Thrombin Using a US-Guided, American Journal of Medicine and Medical Sciences, Vol. 10 No. 12, 2020, pp. 934-939. doi: 10.5923/j.ajmms.20201012.02.
1. Introduction
- The frequency of femoral artery pseudoaneurysm caused by vascular access is increasing due to the development and widespread use of minimally invasive percutaneous endovascular procedures. Symptoms include pain, inguinal swelling, skin necrosis, and compression of the surrounding organs. If left untreated, the pseudoaneurysm can rupture and cause hemodynamic damage. [1-3] Treatment options range from simple observation to surgery, ultrasound-guided compression, US-guided thrombin injection, and endoluminal management. [3] This study aimed to review the success and complication rates, as well as factors associated with technical failure regarding the percutaneous management of femoral artery pseudoaneurysms over a 10-year period.
2. Methods
- Patients and diagnostic toolsWe retrospectively reviewed the medical records and radiologic studies of 37 patients who developed post-catheterization femoral pseudoaneurysm and were treated in the Department of Radiology at our institution between March 2009 and June 2019. This research was approved by the Institutional Review Board. Characteristics of the patients and the lesions, the method of treatment used, and the treatment outcomes were analyzed. The diagnosis of femoral pseudoaneurysm was made using either Doppler US or contrast-enhanced computed tomography (CT). Thirty-two patients were treated with US-guided percutaneous thrombin injection, and five patients were treated using an endovascular technique. US-guided thrombin injectionThe sonographic diagnosis of pseudoaneurysm was made using gray-scale, color Doppler, or power Doppler sonography. “Yin-yang” and “to-and-fro” are characteristic color Doppler signs, which occur as the arterial blood flow enters and exits the pseudoaneurysmal sac. If a pseudoaneurysm was found on the US, patients underwent a detailed evaluation of the origin artery, diameter of the pseudoaneurysmal sac, diameter of the neck, and the presence of multiloculation. Before starting the procedure, the skin in the inguinal area was sterilized and covered with a sterile drape. The US transducer was also covered with a sterile sleeve. Thrombin lyophilized powder (5000 IU) was mixed with 5 cc of normal saline to make a 1000 IU/cc solution. This solution was then transferred to a 1cc syringe and connected to a 19- or 22- gauge needle. Under US guidance, the needle tip was inserted into the neck of the pseudoaneurysmal sac. Then, about 1 cc of thrombin solution was injected into the pseudoaneurysmal sac, and care was taken to ensure the thrombus did not come out of the sac. Thrombin was continuously injected until the pseudoaneurysm became close to being fully thrombosed and the color Doppler flow disappeared. Although the sac was not completely excluded, it was expected that it would be fully thrombosed upon follow-up examination. When the pseudoaneurysmal sac was multiloculated, thrombin was injected into the proximal locule closest to the origin artery. This is because if the proximal locule was blocked, there would be no blood flow to the distal locules, resulting in spontaneous thrombosis. After the needle was removed from the pseudoaneurysm, an US was used to confirm that there was no thrombosis around the origin artery or distal peripheral arteries. Patients were instructed to rest for about 12 to 24 hours after the procedure.The patients were followed up with using either US or contrast-enhanced CT within 1 month. Complete thrombosis was defined as the complete exclusion of the aneurysm during the injection and follow-up examination. Partial thrombosis was defined as residual blood flow in the sac upon follow-up examination.Statistical analysisStatistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) statistical software, Version 22.0. Categorical variables between patients who underwent complete and partial thrombosis were compared using Pearson’s chi-square test, and continuous variables were compared using logistic regression analysis. P-values less than 0.05 were considered statistically significant.The location and origin of pseudoaneurysm were considered to be categorical, and were coded as either right or left, and originating from either the common or superficial femoral artery, respectively. Platelet count (normal or low) was also analyzed as a categorical variable. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were analyzed as categorical variables and coded as normal or elevated. Normal laboratory values were determined according to the standards of our hospital (normal platelet level, 130 K/uL; PT, 1.05 INR; aPTT, 33.2 seconds). Whether patients were taking antithrombotic agents (antiplatelet agents or anticoagulants) at the time of pseudoaneurysm treatment (yes/no) was analyzed as a categorical variable. Body mass index (BMI) was also analyzed as a categorical variable, and was coded as normal verses overweight (normal value < 25.0 kg/m2, World Health Organization). Other categorical variables included sex, multiloculation, hypertension, diabetes, and end-stage renal disease. The continuous variables were age (years), interval between causative procedure and treatment (days), greatest dimension of pseudoaneurysm sac (mm), neck width of the pseudoaneurysm (mm), and the amount of injected thrombin (IU).
3. Results
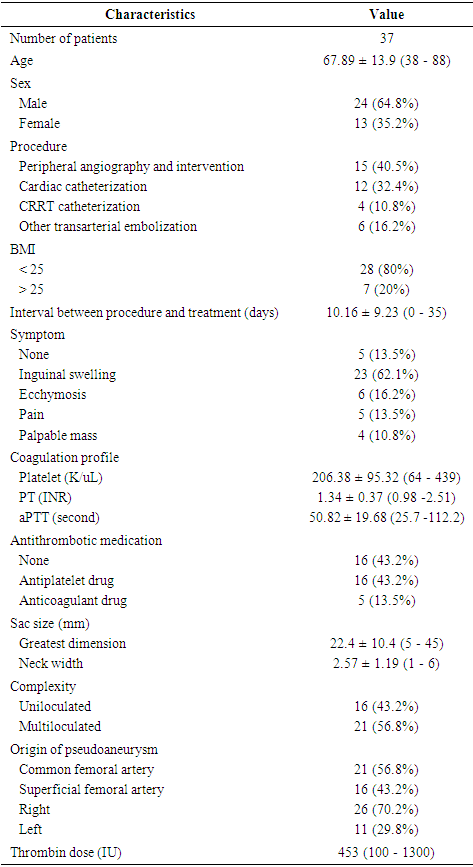
- A total of 37 patients with a mean age of 67.9 ± 13.9 years (range = 38-88 years) had their post-catheterization pseudoaneurysms treated by US-guided thrombin injection or endovascular management. The characteristics of the patients and their pseudoaneurysms are shown in (Table 1). Twenty-four patients were males and 13 were females. The clinical presentations of post-catheterization pseudoaneurysms included inguinal swelling, ecchymosis, pain, and a palpable mass with or without bruit. Five patients had no symptoms and their pseudoaneurysms were found by chance while undergoing contrast-enhanced CT for a separate purpose. Fifteen patients developed a femoral pseudoaneurysm after peripheral angiography and intervention (40.5%), 12 patients following cardiac intervention (32.4%), 6 patients following transarterial embolization of internal organ bleeding (16.2%), and 4 patients following continuous renal replacement therapy (CRRT) application (10.8%). Twenty-one patients (56.7%) were receiving antithrombotic therapy including antiplatelet or anticoagulant drugs at the time of the procedure. Sixteen patients (43.2%) were being treated with antiplatelet agents such as aspirin or clopidogrel, and five patients (13.5%) were being treated with anticoagulants such as heparin or warfarin. Six patients had a low platelet count (< 130 K/uL). The pseudoaneurysm arose from the right side in 26 patients (70.2%), the left side in 11 patients (29.8%), the common femoral artery in 21 patients (56.8%), and the superficial femoral artery in 16 patients (43.2%). The aneurysm was uniloculated in 16 patients (43.2%) and multiloculated in 21 patients (56.8%). The average greatest diameter of the pseudoaneurysmal sac was 22.4 mm (range = 5 - 45 mm) and the average neck width was 2.57 mm (range = 1 - 6 mm). The average time between femoral catheterization and treatment was 10.1 days (range = 0-35 days).
|
|
|
|
4. Discussion
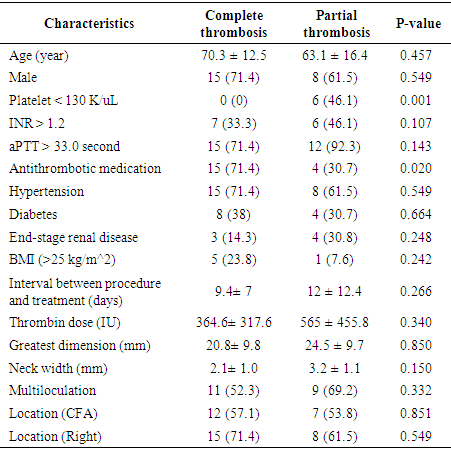
- Treating iatrogenic pseudoaneurysms with an injection of thrombin using a US-guided approach is now widely used due to several advantages including high success rates, promptness of the procedure, minimal inconvenience to the patient, and low complication rates. [4,5] The reported success rates of US-guided thrombin injections range between 85 and 100%. [5-14]. In our study, the success rate was 66.6%, which is relatively low compared to other studies. However, in some studies, the success rate includes patients who had complete thrombosis not only after the first injection, but also after the second or third injection. For example, Khoury et al. [12] and Paulson et al. [13] reported success rates of 96.1% and 96.4%, respectively, but the success rates after the first injection were 88% and 90%, respectively. In a study by Gurel et al. [8], the overall success rate was 96.4%, which included secondary injections, supplemental compression, and spontaneous occlusion at follow up. Only 69% of patients had complete thrombosis after a single injection, which is similar to the results of our study (66.6%). If our study included patients who had spontaneous occlusion at follow-up or had complete thrombosis after the second injection, and excluded patients who were not followed up with, the success rate would be 85% (23 out of 27 patients). Nevertheless, our success rate is relatively low compared to other studies, which could be due to a lack of technical experience at our institution. Since the management was performed without using an established institutional protocol, there is room for improvement. Being excessively cautious when practicing in order to avoid distal embolization (even though complete thrombosis was not achieved) may have also contributed to the low success rate. Achieving complete thrombosis was not a priority because spontaneous occlusion of the remaining aneurysmal sac at follow-up was to be expected. Even if the sac remained at follow-up, it was not difficult to perform an additional thrombin injection. Therefore, we were not set on achieving complete thrombosis at a first injection. In addition, we injected thrombin from the tip of the needle near the neck of the pseudoaneurysm. We chose this location to try to spread the thrombus evenly within the sac, which is formed by contact between the bloodstream coming through the neck and the thrombin. However, in other studies, the needle tip was placed in the center of the pseudoaneurysmal sac [3,8-10,14,15] or away from its neck [1,13,16], to avoid distal embolization. Our study also did not have any limb ischemic event. The difference in success rates between our study and previous studies may be due to methodological differences. So further study regarding this is needed.In the partial thrombosis group, the number of patients with thrombocytopenia (< 130 k/uL) was significantly higher compared to the complete thrombosis group (P=0.01), suggesting that percutaneous pseudoaneurysm treatment is less likely to be successful in patients with a low platelet account. Esterson et al [7] reported that thrombocytopenia was an independent predictor of pseudoaneurysm recurrence, which is similar to our study. Many studies insist that antithrombotic therapy does not affect the success rate of thrombin injections [4,10,13,17,18]. In our study, we found that patients using antithrombotic medication were likely to achieve complete thrombosis (P= 0.02). However, since this finding does not make sense, and because we could not find a study that had similar results, we believe that the effect is a result of the small sample size used in our study.Shieman et al. [5] reported no significant differences in the maximum dimensions or neck diameters of pseudoaneurysms, or between thrombin doses when comparing successful treatments and failures. Gurel et al. [8] reported no statistically significant differences in pseudoaneurysm size or number of locules. Together, these studies were consistent with our findings. Specifically, we found no significant associations between partial thrombosis and the greatest diameter or neck width of the pseudoaneurysm, amount of injected thrombin, or multiloculation of the pseudoaneurysm. However, Vlachou et al. [9] reported that a pseudoaneurysm size less than 2 cm was predictive of the procedural success, and Esterson [7] reported that a pseudoaneurysm size larger than 2 cm was a significant predictor of pseudoaneurysm recurrence. Also, Chen et al. [1] and Krueger et al. [19] reported greater rates of incomplete thrombosis with multilocular pseudoaneurysms. However, our study found no association between partial thrombosis and pseudoaneurysm size or multiloculation.Madaric et al. [20] found that pseudoaneurysm recurrence found at the 1 month follow-up was associated with obesity (BMI>30). Our study did not show a significant relationship between obesity (BMI>25) and partial thrombosis, however, only two patients who had a BMI above 30, making it difficult to compare statistically.Frequently used endovascular techniques include coil embolization and covered stent placement, which both have good success rates and are safe to perform. [21,22] Another endovascular techniques is percutaneous thrombin injection, while inflating a balloon catheter across the neck of the pseudoaneurysm. [23] Although useful in treatment, these endovascular techniques have disadvantages, including increased cost, time, invasiveness, and technical difficulties. Additionally, since these techniques are usually performed through the contralateral artery, they may result in contralateral femoral artery complications. Patients who receive coil embolization or stent placement in the femoral artery often experience discomfort while walking after procedure. [4,16] Thalhammer et al. [22] reported that during the 1 year follow-up of 23 patients who underwent endovascular covered stent placement for an iatrogenic pseudoaneurysm, four patients (17%) had stent thrombosis. Therefore, the two patients who had stent deployment in our institution need to be followed up.Aside from five patients in our study who experienced pain at the puncture site, there were no complications. Therefore, this procedure is considered to be safe. Geofferey et al. [11] found that the overall complication rate of US-guided thrombin injection was as low as 1.3%. The most serious procedure-related complication is downstream arterial thrombosis resulting in limb ischemia, which we did not experience. Similar to our study, Vlachou et al. [9], Tomasz et al. [6], and Mishra et al. [24] did not experience any limb-ischemic events during or after pseudoaneurysm management. Other reported complications include venous thrombosis, pulmonary embolism, allergic or anaphylactic reaction, vasovagal reaction, and rarely skin necrosis [3,11,13], which we also did not experience.One of the limitations of the present study was that it was a small, retrospective study. Additionally, as the research extended over a decade, many patients were not followed up with, and some data was unavailable. Further, we were unable to assess long-term recurrence rates or complications that occurred after the initial follow-up.In conclusion, US-guided thrombin injection is considered an initial treatment option for a femoral pseudoaneurysm caused by vascular access, due to its simplicity and success rate. In cases where it is difficult to treat with thrombin injection alone, endovascular treatment such as stent-graft placement may be helpful. Low platelet count affects the success rate of the percutaneous management of post-catheterization pseudoaneurysms.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML