Navruzova Sh. I., Ganieva Sh. Sh., Jurayeva F. R.
Department of Pediatrics Bukhara State Medical Institute, Uzbekistan
Correspondence to: Navruzova Sh. I., Department of Pediatrics Bukhara State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The authors conducted a study of immunological and biochemical parameters in biological environments in children with food allergies. In studies in patients with Helicobacter pylori associated gastrointestinal allergy was established a strong positive relationship of Helicobacter pylori infection with saliva sIgE (r=0.83), blood IgE (r=0.71) and blood IgM (r=0.56), which was not typical for gastrointestinal allergies without Helicobacter pylori infection.
Keywords:
Helicobacter pylori, Gastrointestinal food allergy, Correlation
Cite this paper: Navruzova Sh. I., Ganieva Sh. Sh., Jurayeva F. R., Diagnostic Value of Immuno-Biochemical Tests for Gastrointestinal Allergies in Children, American Journal of Medicine and Medical Sciences, Vol. 10 No. 11, 2020, pp. 901-904. doi: 10.5923/j.ajmms.20201011.14.
1. Introduction
Despite the fact that gastrointestinal food allergy is becoming an increasingly urgent problem in Pediatrics, and, according to various researchers, allergic lesions of the gastrointestinal tract occur in 25-50% of patients with such a common pathologies such as allergies to cow's milk proteins, these conditions remain a "white spot" for many pediatricians [1,4,5,12,13,18].The frequency of food allergies in children is 8.5% of all gastrointestinal diseases. The structure of food аllergy is dominated by acute urticaria - 53.3% and gastrointestinal аllergy - 21.7%. in 37.8% of cases of food аllergy, comorbidity is established. At the same time, food аllergy is more often combined with anemia-56.6% and acute respiratory diseases-28.4%, and is mainly manifested by the respiratory form [6].Gastro intestinal symptoms caused by food intake and related to immediate reactions (nausea, vomiting, abdominal pain, diarrhea), occur immediately after the use of a causally significant product or within the first 2 hours (for diarrhea, it is possible a little later). In addition to the above symptoms, isolated signs such as anxiety after eating, copious regurgitation, refusal to eat occur in young children [12].The variety of factors that cause the development of allergic diseases, the complex pathogenesis, and the uneven response of patients to therapy have become the basis for the identification of phenotypes and endotypes of allergic diseases. Such a high interest in this problem is due to the fact that modern advances in the diagnosis and treatment of allergic diseases do not always satisfy patients, and many of them are marked by the inefficiency of standard treatment [11].Currently, it is extremely difficult to make a differential diagnosis between non-IgE-mediated gastrointestinal allergic manifestations and functional disorders of the gastrointestinal tract. Involvement of more than one system in the pathological process may indicate a greater probability of allergic damage [10].Available tools for allergy diagnostics, including in Pediatrics, are the evaluation of the effectiveness of elimination measures, in vivo (skin tests) and in vitro testing to determine the level of allergen-specific IgE antibodies circulating in the blood serum [16].The content of IgE, determined using different laboratory methods, may vary, so the data on the clinical value of test results should be necessarily "linked" to a specific method. It was found that the younger the child's age, the lower the concentration of specific IgE, which corresponds to an obvious reaction to food [3].Studies have evaluated the relationship between eosinophilic esophagi is and H. pylori infection, but the feedback has not been confirmed. High H. pylori resistance and, consequently, low eradication rates are still one of the main risk factors for the development of the disease [14].There is evidence that laboratory tests used to diagnose H. pylori in patients with functional dyspepsia increased the cost of medical care in the group with a high incidence of the disease, while the symptoms of dyspepsia decreased against the background of eradication therapy. Diagnostic tests for H. pylori infection in children, are usually not recommended in the absence of alarming signs or symptoms, which proves the need to develop new non-invasive methods for diagnosing H. Pylori infection [17].The higher prevalence of H. pylori among patients with allergic diseases, observed in some studies, raised the question of the role of inflammation (observed in the presence of H. pylori infection) in the development of allergies. A positive association between H. pylori and allergic diseases is still being discussed in relation to urticaria. In addition, the international guidelines on urticaria state that in some cases of chronic spontaneous urticaria, the eradication of infections such as H. pylori, intestinal parasites, and bacterial infections of the nasopharynx have been shown to provide an advantage in the treatment of this disease [7,8].Studies have shown that H. pylori infection is pathogenetically associated with allergic diseases, with a suspected pathogenetic mechanism that H. pylori infection can provoke the release of histamine from basophils. Although the theoretical background remains uncertain, hypothetically H. pylori appears to suppress the allergic response of the immune response through T-helper cells. Thus, the results obtained contradict each other, suggesting that infection H. pylori is associated with the risk of developing allergies and is also a protective factor against allergies [15].
2. The Purpose of the Study
The purpose of the study was to study the relationship between immunological and biochemical parameters of blood and saliva in the gastrointestinal form of food allergy (FA) in children, depending on H.pylori infection.
3. Materials and Methods of Research
63 sick children from 3 to 10 years of age with gastrointestinal allergy (GIA), who were undergoing inpatient examination and treatment at the BRCMPMC were examined to study the effect of concomitant pathology on the course and prognosis of food allergy (FA). The sick children were divided into 2 groups: 32 children with H. pylori-associated GIA and 31 children with H. pylori-unassociated GIA. All patients were examined for general, biochemical blood tests (bilirubin, ALT, AST, urea, diastase) and urine (general analysis of urine, diastase in urine). H. pylori in the blood was determined by the ELISA method (Human, Germany) Immunological studies were performed by studying humoral immunity indicators (IgA, IgM, sIgA, IgE) and cytokines (IL-8, TNF-α) in peripheral blood serum and saliva by the ELISA method (Vector Best JSC, Russia), according to the attached instructions.Statistical analysis of the obtained results was carried out using the methods of variation statistics. The reliability of differences in average values was evaluated based on the Student's test (t) with the calculation of the probability of error (P) when checking the normality of the distribution and equality of general variances (f – Fisher's test). The data was considered reliable if t ≥2 and P<0.05.Correlation analysis was carried out using the methods of Spearman (Rs) and Pearson (r). Criteria evaluated on a scale of Chedoke: 0.1 < r < 0.3: weak; 0.3 < r < 0.5: moderate; 0.5 < r < 0.7: noticeable; 0.7 < r < 0.9: high; 0.9 < r < 1: very high.
4. Results and Discussion
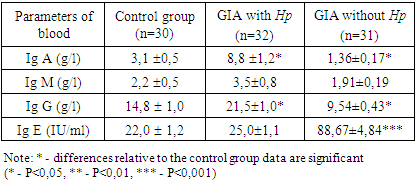
The study of factors of humoral blood immunity in patients with GIA showed a characteristic imbalance in the composition of immunoglobulins in FA depending on H. pylori infection (table 1).Table 1. Concentration of immunoglobulins in children with food allergies( M±m)
 |
| |
|
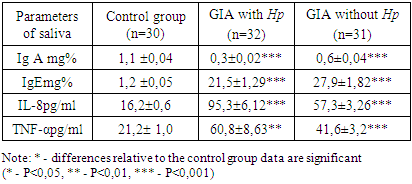
Analysis of the blood test results of patients with GIA associated with H.pylori showed a significant increase in the concentration of Ig A-8.8 ± 1.2 g / l versus 3.1 ± 0.5 g/l in the control and IgG-21.5±1.0 g/l versus 14.8 ± 1.0 g / l in the control (P<0.05).Comparative characteristics of the content of immunoglobulins in the blood of patients with GIA without association with H. pylori revealed opposite shifts in the studied immunoglobulins. There was a significant decrease in the concentration of IgA to 1.36±0.17 g/l and IgG to 9.54±0.43 g / l (P<0.05) in comparison with the data of the control group.The IgM concentration had an unreliable tendency to increase relative to the control in GIA associated with H. pylori and vice versa, in GIA without association with H. pylori, it tended to decrease against the background of a significant increase in the level of Ig E-88.67±4.84 IU/ml versus-22.0 ± 1.2 IU/ml in the control.In the study, patients with GIA without association with H. pylori showed a significant 4-fold increase in Ig E-88.67±4.84 mcl in relation to control parameters-22.0 ± 1.2 mcl (P<0.001).The study of secretory immunity in patients with GIA showed a significant decrease in sIgA in both groups of observation (table 2).Table 2. Concentration of immunoglobulins and cytokines in saliva in children with food allergies (M±m)
 |
| |
|
There was a significant decrease in sIgA by 3.7 times (0.3 ± 0.02 mg%) in GIA associated with H. pylori and by 1.83 times (0.6±0.04 mg%) in GIA without association with H. pylori relative to the control parameters - 1.1 ± 0.04 mg% (P<0.001).The concentration of sIgE increases both when GIA is associated with H. pylori (up to 21.5±1.29 mg%) and when it is absent (up to-27.9±1.82 mg%) versus control-1.2 ± 0.05 mg% (P<0.001).In order to introduce non-invasive manipulations in the practice of pediatric allergy and improve methods of non-invasive diagnosis of FA in children, were studied cytokines in saliva. The results showed a significant increase in the level of IL-8 and TNF-α in saliva in GIA in children, regardless of H. pylori infection.It is known that IL 8 is produced by monocytes, fibroblasts and endothelial cells. It acts as a neutrophil activator, since it is a chemokine, an endogenous chemoattractant. It stimulates the directed movement of various types of white blood cells [9].IL-8, TNF-α, and other cytokines produced by macrophages during an early inducible response are pro-inflammatory cytokines. Their action completely determines the development of the inflammatory process that develops when the antigen is introduced into the macroorganism [2].The study found an increase in IL-8 by 5.8 times (95.3±6.12 pg/ ml) in GIA associated with H. pylori and by 3.53 times (57.3±3.26 pg/ml) in GIA without association with H. pylori in relation to control - 16.2 ± 0.6 pg/ml.Tissue macrophages, having absorbed microbes, activate and synthesize cytokines, in particular tumor necrosis factor α (TNF-α) [9].There was a significant increase in the concentration of TNF-α in saliva in patients with GIA, regardless of H. pylori infection. In GIA associated with H. pylori, TNF-α increases to - 60.8±8.63 pg/ml, and in GIA without association with H. pylori-to 41.6±3.2 pg/ml against control-21.2 ± 1.0 pg/ml (P<0.01).Correlation analysis helps to predict the possible values of one indicator, knowing the value of another, which is very important when performing non-invasive diagnostic manipulations with high accuracy and significance.During the period of acute FA in patients with H. pylori-unassociated GIA, the linear correlation coefficient showed a strong positive relationship between blood and saliva IgE (r=0.80) and a strong inverse relationship between saliva sIgA and blood IgE (r=-0.78) (Fig. 1).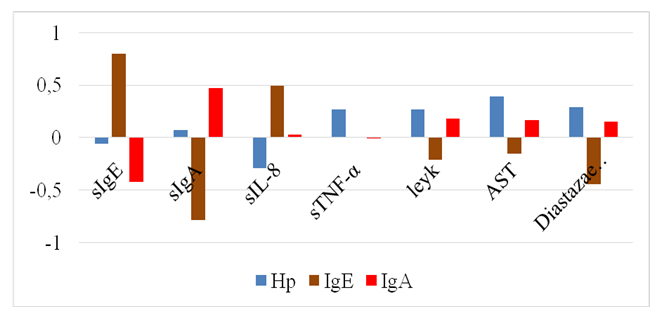 | Figure 1. Correlations of immuno-biochemical parameters in H. pylori unassociated GIA |
Based on the results of the study, it can be stated that an increase in the concentration of IgE in both blood and saliva is accompanied by a decrease in the level of sIgA in saliva in children with GIA. The established relationships between saliva and blood immunoglobulins allow early and accurate non-invasive diagnosis of GIA, which, due to the presence of a correlation, is sufficient to study sIgE in saliva.It is known that IL-8 is a pro-inflammatory cytokine and plays a huge role in the innate immune system. IL-8 stimulates the secretion of histamine by basophils and is one of the stimulators of angiogenesis [5]. In our studies, patients with Helicobacter pylori unassociated GIA showed an average positive relationship between saliva IL-8 and blood IgE (r=0.49), between saliva IL-8 and saliva sIgE (r=0.39). There is also an average negative feedback in IL-8 saliva with sIgA (r=-0.45). Consequently, in saliva, an increase in IgE is accompanied by an increase in IL-8 and a decrease in sIgA.Studies on Helicobacter pylori infection in GIA have shown a weak negative association of IL-8 saliva with Helicobacter pylori blood (r=-0.29).Of interest was the study of the relationship between blood H. pylori and other blood parameters in GIA. In this regard, weak positive relationships were found between the content of H. pylori and white blood cells (r=0.27), urine diastase (r=0.29), and TNF-α of saliva (r=0.27). The average positive association of H. pylori in blood with AST of blood was also detected (r=0.39). At the same time, an average positive association ofurine diastase with saliva sIgA (r=0.40), a negative association with saliva sIgE (r=-0.50) was also detected.It should be noted that there is a positive relationship between saliva and blood IgA (r=0.47), while saliva sIgA has a strong negative relationship with blood IgM (r=-0.59).The established pattern makes it possible to predict H. pylori infection by the level of diastase in the urine, which proves that it is possible to limit non-invasive diagnostics while reducing the financial costs of tests.In studies in patients with H. pylori associated GIA, a strong positive relationship of H. pylori infection with saliva sIgE (r=0.83), blood IgE (r=0.71) and blood IgM (r=0.56) was established, which was not typical for GIA without H. pylori infection. At the same time, H. pylori retains a weak positive relationship with blood IgA (r=0.25) and a weak negative relationship with saliva IgA (r=- 0.31) (Fig. 2).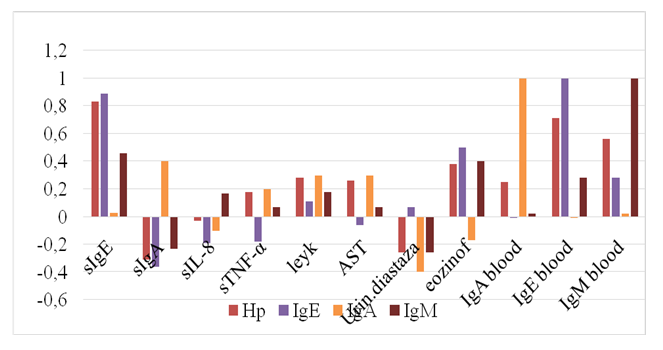 | Figure 2. Correlations of immuno-biochemical parameters in H. pylori associated GIA |
The characteristic moderate positive relationship of H pylori blood with blood leukocytes (r=0.28), blood eosinophils (r=0.38) and with blood AST (r=0.26) was also established in the presence of a moderate negative relationship with urine diastase (r=- 0.26). In contrast to the correlations in H. pylori unassociated GIA, when GIA is associated with H. pylori infection, there is an average positive association of TNF-α saliva with IL-8 saliva (r=0.44) and a weak positive association of TNF-α saliva with blood IgA (r=0.20).
5. Conclusions
Therefore, in studies on the correlation of immuno-biochemical parameters, it was found that saliva sIgE and IL-8 are more informative indicators of secretory immunity and saliva cytokines for urgent and non-invasive diagnostics of GIA.Thus, for early and non-invasive diagnosis and differentiation of GIA in children, depending on Helicobacter pylori infection, it is considered appropriate to determine the concentration of secretory sIgE and diastase in the urine.
References
| [1] | Antichelicobacter therapy in children with chronic urticaria N. V. Malyuzhinskaya, O. V. Polyakova, S. A. Yemelyanova, S. V. Smykova, N. V. Nikolenko// Volgograd scientific and medical journal 2/2016/c / 40-42. |
| [2] | Becker T. C. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells / T. C. Becker, S. M. Coley, E. J. Wherry, R. Ahmed / / J. Immunol – - 2005. - Vol. 174. - P. 1269-1273. |
| [3] | Cheburkin A. A. Diagnostics of allergic and non-allergic forms of food intolerance in children// Questions of modern Pediatrics / 2013 / Volume 12 / no. 2 C44-51. |
| [4] | Dmitrienko M. A., Ginak A. I. Biotechnological model of the formation of gaseous biomarkers in the persistence of Helicobacter pylori infection // University news. Applied chemistry and biotechnology. 2017. Vol. 7, N 1. C. 1 33-1 39. DOI: 10.21285/2227-2925- 2017-7-1 -133-1 39. |
| [5] | Eshmolov S. N., Sitnikov I. G., Melnikova I. M. cytokines TNF-α, IFN-γ, IL-1, IL-4, IL-8 and their role in the immune response in infectious CNS damage in children // Children's infections. - 2018. - Volume 17, No. 1. - Pp. 17-22. |
| [6] | Ganiyeva Sh. Sh., Jurayeva F. R., Navruzova Sh.I., Features of the frequency of gastrointestinal allergies in children// J. New day in medicine- 2 (30).- 2020. –p.-335-337. |
| [7] | Helicobacter pylori Infection and Risk Factors in Relation to Allergy in Children Ilva Daugule, Daiga Karklina, Silvija Remberga and Ingrida Rumba-Rozenfelde. https://doi.org/10.5223/pghn.2017.20.4.216. Pediatr Gastroenterol Hepatol Nutr 2017 December 20(4): 216-221 pISSN: 2234-8646 eISSN: 2234-8840. |
| [8] | Ilva Daugule, Jelizaveta Zavoronkova, and Daiga Santare Helicobacter pylori and allergy: Update of research World J Methodol. 2015 Dec 26; 5(4): 203–211. Published online 2015 Dec 6. doi: 10.5662/wjm.v5.i4.203. |
| [9] | Khaitov RM Immunology: textbook for universities / RM Khaitov. - 2nd ed., reprint. and add. - M.: Geotarmedia, 2013. - 521 with Il. + 1 CD. |
| [10] | Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012; 55(2): 221–229. doi: 10.1097/MPG.0b013e31825c9482. |
| [11] | Larkova I. A., Revyakina V. A. the Problem of food allergies and elimination diets in children // Nutrition issues. 2015. Vol. 84, No. 5. Pp. 49-50. |
| [12] | Lee SY. Future candidates for indications of Helicobacter pylori eradication: do the indications need to be revised? J Gastroenterol. Hepatol 2012; 27: 200–11. |
| [13] | Makarova S. G., Namazova-Baranova L. S., Vishneva E. A., Ereshko O. A., Gordeeva I. G. Gastro-intestinal food Allergy in children. Questions of modern Pediatrics. 2017; 16 (3): 202–212. doi: 10.15690/vsp. v16i3.1730. |
| [14] | Mišak Z, Hojsak I, Homan M. Review: Helicobacter pylori in pediatrics. Helicobacter. 2019; 24 (Suppl.1): e12639. https://doi.org/10.1111/hel.12639. |
| [15] | Sang Pyo Lee,Sun-Young Lee,Correlation Between Helicobacter pylori Infection, IgE Hypersensitivity, and Allergic Disease in Korean Adults 2014 Helicobacter ISSN 1523-5378. doi: 10.1111/hel.12173. |
| [16] | Snovskaya M. A., Batyrova A. S., Namazova-Baranova L. S., Alekseeva A. A., Vishneva E. A., Kozhevnikova O. V., Marushina A. A., Lubov V. N. on minimizing the cost of highly effective Allergy diagnostics in children: analysis of the consistency of the results of allergological in vitro and in vivo examinations. Bulletin of the RAMS. 2015; 70 (6): 748-755. Doi: 10.15690/vramn583. |
| [17] | Ünlüsoy Aksu A, Yılmaz G, Eğritaş Gürkan Ö, Sarı S, Dalgıç B. The effect of Helicobacter pylori eradication on functional dyspepsia in Turkish children. Helicobacter. 2018; e12497. https://doi.org/10.1111/hel.12497. |
| [18] | Vandenplas I., Zakharova I. N., Dmitrieva Yu. a. Gastrointestinal manifestations of food allergies or functional disorders of the gastrointestinal tract principles of differential diagnosis and diet therapy medical Council • no. 16, 2016 with 110-114. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
