Murotov Temur Malik Nizomovich1, Avakov Vyacheslav Ervandovich2, Ismoilov Baxodir Yuldoshovich3, Jumabaev Ruslan Rashidovich4
1Basic Doctoral Student of the Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
2Doctor of Medical Sciences, Professor of Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
3Assistant of the Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
4Master of the Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Republic of Uzbekistan
Correspondence to: Murotov Temur Malik Nizomovich, Basic Doctoral Student of the Department of Anesthesiology and Resuscitation of the Tashkent Medical Academy, Tashkent, Republic of Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Severe TBI leads to cerebral edema, increased ICP, resulting in decreased cerebral perfusion pressure and increased secondary ischemic injury. Appropriate and timely treatment of elevated ICP improves cerebral perfusion to reduce this secondary ischemic stroke. Mannitol is currently a widely used diuretic for the treatment of ICH and is recommended in guidelines for the treatment of TBI in adults. The aim of the study: to study the effect of early prescription of 15% mannitol solution on the studied parameters of systemic, central hemodynamics, homeostasis, ICP and CPP, to determine its effectiveness and safety in the adult population with TBI. Research methods: A total of 30 patients were included in this study, conducted over 3 years (2017-19) in the departments of neurosurgery and anesthesia and resuscitation of the TMA. Conclusion: therefore, it can be concluded that the rate of infusion may depend on the duration of the action of mannitol, because the faster the infusion, the greater the likelihood of termination of the effect due to rapid excretion of mannitol by the kidneys or its penetration into the brain tissue.
Keywords:
Intracranial pressure, Cerebral perfusion pressure, Traumatic brain injury, Mannitol
Cite this paper: Murotov Temur Malik Nizomovich, Avakov Vyacheslav Ervandovich, Ismoilov Baxodir Yuldoshovich, Jumabaev Ruslan Rashidovich, Assessment of the Effect of Mannitol on Intracranial Pressure, Cerebral Perfusion Pressure, Systemic and Central Hemodynamics in Patients with Isolated Traumatic Brain Injury, American Journal of Medicine and Medical Sciences, Vol. 10 No. 11, 2020, pp. 870-875. doi: 10.5923/j.ajmms.20201011.08.
Traumatic brain injury (TBI) is the leading cause of death and disability worldwide, accounting for about 30% of all injury-related deaths [1]. After primary cerebral injury, management of TBI focuses on the prevention and treatment of secondary brain damage, including control of intracranial pressure [2]. Severe TBI leads to cerebral edema, increased ICP, resulting in decreased cerebral perfusion pressure and increased secondary ischemic injury. Appropriate and timely treatment of elevated ICP improves cerebral perfusion to reduce this secondary ischemic stroke. The use of hyperosmolar agents to lower ICP has been known for over 90 years, but it continues to lack evidence. Serum osmolarity increases with the introduction of hyperosmolar solutions, extracting fluid from the interstitial space of the brain into the vascular bed and leading to a decrease in the volume of brain tissue and, as a result, to a decrease in ICP [3,4]. Mannitol is currently a widely used diuretic for the treatment of ICH and is recommended in guidelines for the treatment of TBI in adults [5]. Although recent TBI treatment guidelines have not reported any evidence to support or refute the use of mannitol in the treatment of ICH [6], there are no published guidelines or protocols that define the timing, concentration, or route of administration of mannitol. in the treatment of severe TBI [7]. The decision as to whether to use mannitol, GSR, or the combination is left to the discretion of the clinician.
1. The Aim of the Study
To study the effect of early prescription of 15% mannitol solution on the studied parameters of systemic, central hemodynamics, homeostasis, ICP and CPP, to determine its effectiveness and safety in the adult population with TBI. All patients in this group had severe isolated TBI, mainly traffic accidents and falls.After assessment on the Glasgow coma scale, all patients underwent a CT scan of the head for diagnostic purposes and to exclude the need for emergency neurosurgical intervention. Inclusion criteria were age> 18 years, isolated TBI, GCS< 8, sustained increase in ICP> 20 mm Hg. for more than 5 minutes.Exclusion criteria were: polytrauma, oliguria, renal failure, hemoglobin< 8 g / L, serum osmolarity> 320 mOsm / L, use of hypertonic saline solutions in the previous 6 hours.
2. Research Methods
A total of 30 patients were included in this study, conducted over 3 years (2017-19) in the departments of neurosurgery and anesthesia and resuscitation of the TMA. All patients were provided with analgesia and, if necessary, sedative therapy when the patients were agitated (dormicum, diazepam). Vasoactive support (norepinephrine) was administered to patients with arterial hypotension. Insulin was used to maintain glycemia at 6-8 mmol / L. A set of variables was collected for each patient, which included data on demographic characteristics, baseline GCS. ICP was monitored with the possibility of manometry with subarachnoid lumbar puncture and M-echopulsogram with the Kompleksmed 1.2 apparatus.Other monitoring included indications of systemic (BP, SBP, heart rate) and central hemodynamics (II, HI, TPVR), plasma osmolarity, sodium level, blood hemoglobin, hematocrit.When ICP exceeded 20 mm Hg. over 5 min, a bolus of 15% mannitol was administered through the central venous line at a rate of 6-8 ml / min (120-130 drops / min). The infusion was stopped when the ICP dropped below 20 mm Hg.Study stages: start of infusion, after cessation (ICP< 20 mm Hg), 30, 60 minutes after cessation of mannitol infusion.Serum sodium level, osmolarity, Ht were measured before and after therapy.The table below shows the baseline demographic and clinical characteristics of patients in this (I) group.Table 1. Demographic and clinical characteristics of patients in group I (n = 30)
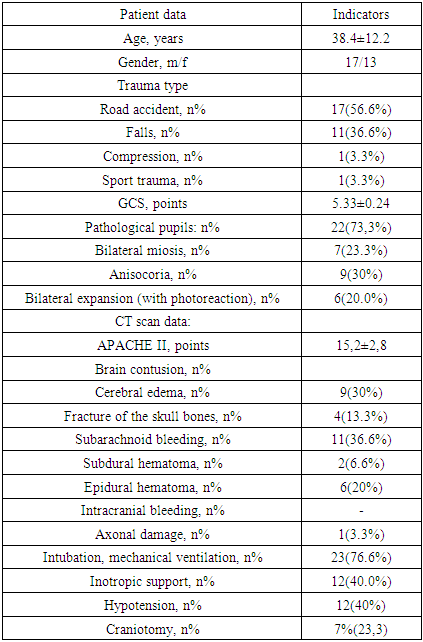 |
| |
|
The age of the patients in this group was in the range of 20-88 years. In 7 victims, vomiting of gastric contents took place, in 3 of them aspiration syndrome was diagnosed, for which sanitation bronchoscopy with lavage of the airways was performed.Consciousness disorder according to the GCS, averaging 5.33 ± 0.24, was in the range of 5-7.The total severity of the condition according to APACHE II averaged 15.2 ± 2.8 points.The table below shows the data of the clinical examination of patients in this group upon admission to the clinic.Table 2. Indicators of clinical blood tests and hemostasis (n = 30) at admission
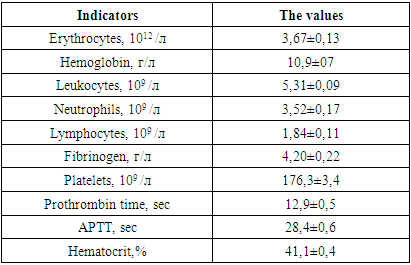 |
| |
|
The presented data indicate moderate anemia of traumatic origin and activation of the blood coagulation system, which is indicated by: a shortening of the prothrombin time by 8.6%, increased fibrinogen values and a decrease in cephalin-coalin time by 8.4% of the lower limit of physiological values of this indicator. All this indicates the activation of the first (formation of prothrombinase) and the second (formation of thrombin) phases of blood coagulation.Systemic and central hemodynamic parameters in patients were also subject to significant changes on admission, as evidenced by the table below.Table 3. Indicators of systemic and central hemodynamics in patients of this group at admission (n = 30)
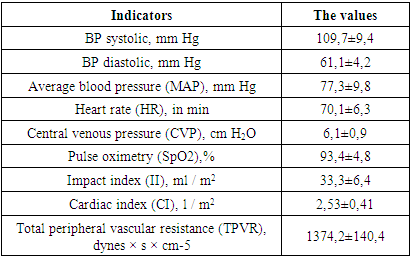 |
| |
|
The averaged values of the studied parameters indicate that the patients of this group on admission had moderate arterial hypotension with a decrease in systolic and diastolic pressure, which affected a decrease in SBP. In 12 patients, due to more pronounced arterial hypotension, inotropic support (norepinephrine, hormone) was used at admission. All this indicated a decrease in the tone of resistive vessels, as evidenced by the parameters of the systemic vascular resistance. They were below the physiological value, which indicated a decrease in tone in the low pressure system (capillaries, venules). The decrease in TPVR was 9.1% of the proper TPVR values during this period (1511.1 dynes × s × cm-5), calculated by us using the following formula:TPVR due (dyn × s × cm-5) = BP av due × 80 / actual MOQThe proper SBP values in the age group of the studied patients are 85 mm Hg.CVP was 25% lower than physiological values. All of the indicated decrease in the one-time and minute performance of the heart, which were at the borderline values of the normo- and hypodynamic circulatory regime, and indicated a deterioration in cerebral circulation.In all patients in this group, upon admission to the ICU, an increase in ICP was recorded, the mean values of which were 27.4 ± 1.9 mm Hg, which explains the initial relative bradycardia (76.1 ± 6.3 per minute). The average values of cerebral perfusion pressure (CPP) were 49.9 ± 5.8 mm Hg, which confirmed the above thesis about the deterioration of cerebral blood circulation.The baseline values for blood electrolytes and plasma osmolarity are shown in the table below.Table 4. Blood biochemical parameters and plasma osmolarity in postulated patients (n = 30)
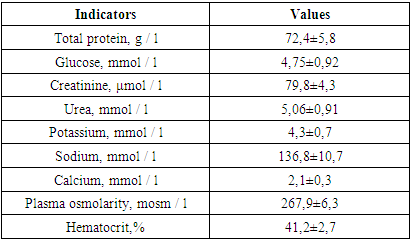 |
| |
|
Analyzing the data presented, it can be noted that all the studied indicators practically did not go beyond the physiological norms for adults. However, it is immediately striking that with insignificant hyponatremia (in terms of mean values), there is a large range in terms of the standard deviation, which made us study the Na + concentration in patients in more detail. Thus, in 11 patients from this group (36.6%), the plasma sodium concentration exceeded 145 mmol, averaging 149.9 ± 4.3 mmol / L, while in the remaining 19 patients (63.4%), the plasma Na + level was below 135 mmol / L, averaging 123.7 ± 6.4 mmol / L. Relative hyponatremia at normal values of glucose and blood urea and led to a decrease in plasma osmolarity by 6.9% of the physiological norm.Table 5. Indicators of systemic and central hemodynamics in the 1st group at the stages of the study
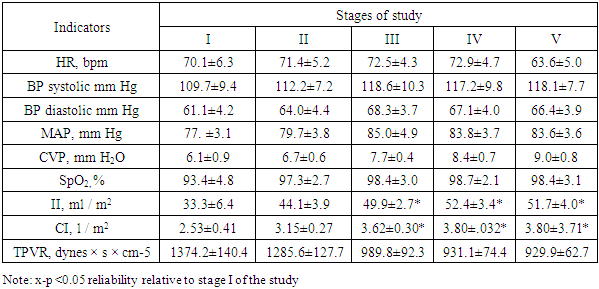 |
| |
|
Baseline heart rate averaged 70.1 ± 6.9 bpm (with a range of 56-87 bpm). No clinically significant changes were observed after a bolus dose of 15% mannitol. There was only a slight tendency to an increase in heart rate, which by 120 minutes was 5% more often than the initial values (p> 0.05). A similar picture was observed with SBP indicators. The maximum increases in SBP were noted by 30 minutes after completion of the mannitol infusion (by 9.9%) relative to the initial values. By the 120th minute, SBP, averaging 83.6 ± 3.6 mm Hg, exceeded the initial values by 8.1%.Improved CVP indices, indicating good venous blood return, and pulse oximetry data.The greatest changes were recorded in terms of indicators of central hemodynamics. Both one-time and minute cardiac performance increased statistically significantly from 30 minutes and after 120 minutes HI and HI were 55.2% and 50.2% higher than the initial values, respectively (p> 0.05).These changes in blood pressure, SBP, II, HI occurred against the background of a significant decrease in the tone of resistive vessels in the low pressure system, indicating an improvement in microcirculation. Thus, TPVR, averaging 1374.2 ± 140.4 initially, after 60 and 120 minutes was lower than these values by 32.3% and 32.5%.The dynamics of ICP and CPP at the stages of the study is reflected in the following table.Table 6. Dynamics of ICP and CPP at research stages (n = 30)
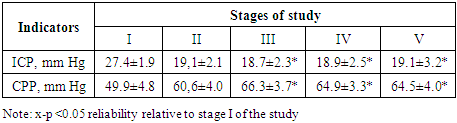 |
| |
|
After a bolus infusion of 15% mannitol, ICP by 30 minutes decreased by a maximum of 31.8% and practically stayed at these figures until 120 minutes of the study (p< 0.05). This decrease in ICP had a positive effect on CPP, which by 30 minutes, averaging 66.3 ± 3.7 mm Hg, was statistically significantly higher than the initial values by 32.8% (p< 0.05).We noted the maximum increase in CPP by 120 minutes by 29.2% relative to the initial values. All of this indicated an improvement in cerebral perfusion. This was facilitated by the hematocrit values, which in the indicated period of time reached 35-37%, as evidenced by the following table.Table 7. Dynamics of blood biochemical parameters after a bolus of 15% mannitol (before and 2 hours after infusion) (n = 30)
 |
| |
|
From the presented data, it is not difficult to notice that mannitol led to hemodilution, due to which the Ht parameter significantly decreased (by 12.4%). As for the concentration of sodium in the blood, it practically did not undergo any changes. Plasma osmolarity, although increased by 10 mOsm / L after mannitol infusion, was not statistically significant.Table 8. A dose of 15% mannitol and the time required to lower ICP below 20 mm Hg
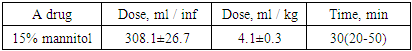 |
| |
|
The average maximum and minimum ICP values obtained in this group (I) of patients were 27.4 ± 1.9 mm Hg. and 18.7 ± 2.3 mm Hg.The average duration of osmotherapy in this group was 7.2 ± 0.4 days (6-8 days).After reaching our goal of treatment (lowering the ICP below 20 mm Hg), the patients were observed for 2 hours, and then every 5-6 hours. The decrease in ICP under the influence of mannitol was accompanied by an increase in urine output to 110-140 ml / h (121.1 ± 9.0 ml). The peak increase in urine output was observed within 30-60 minutes.In 7 (23.3%) patients of this group (3-subdural hematoma and hemorrhage, 4-epidural hematoma), craniotomy with clot evacuation was performed (16.6%). Osmotherapy with mannitol was ineffective for them.Table 9. Dynamics of GCS indicators
 |
| |
|
The stay of patients in ICU in this group averaged 12.6 ± 0.9 days. Stay at the clinic - 19.4 ± 2.1 days. Hospital mortality was 23.3% (7 patients).
3. Discussion
Increased ICP above 20 mm Hg. plays a significant role in the deterioration of neurological status due to impaired cerebral perfusion. According to established treatment guidelines, ICP above 20 mm Hg and CPP below 60 mm Hg are considered critical. Early detection of such critical episodes and the selection of an effective and safe drug for treatment is essential to protect the brain. Osmotherapy has been used since the early 20th century for the treatment of elevated ICP. Intravenous infusion of mannitol is considered the "gold standard" in the treatment of ICH. The generally accepted goal of treatment is to maintain ICP below 20 mm Hg and CPP above 60 mm Hg. Otherwise, the outcome is worse [8].In our study, 15% mannitol at a dose of 7.1 ± 0.3 ml / kg reduces ICP 30 min after bolus administration. Its effect lasts more than 2 hours. These data correspond to those presented in the literature [9,10,11].Due to its low molecular weight (182 Da), mannitol is filtered in the glomeruli and reabsorbed in the nephron as an osmotic diuretic. However, since it is not absorbed, it remains osmotically active in the tubules, which explains its diuretic effect. It does not undergo biotransformation [12]. When administered intravenously, mannitol is distributed mainly in the extracellular sector and excreted in the urine within 24 hours [13]. Pharmacological studies have shown that the plasma half-life of mannitol is 22-24 hours. Its action begins in 15-20 minutes, and the peak effect on the brain occurs 30 minutes after its administration and lasts from 90 minutes to 6 hours [14,15,18]. According to [16,17], mannitol is the main treatment for cerebral edema and ICH in patients with TBI. Nevertheless, there is still no evidence regarding the optimal dose and duration of osmotherapy. The ICP threshold has not been set above which mannitol is recommended.In our study, the duration of osmotherapy with 15% mannitol was within 5-8 days, averaging 6.3 ± 1.2 days, for it is a well-known fact that with repeated and prolonged use, mannitol becomes less effective and can lead to unacceptably high serum sodium levels and plasma osmolarity, which in turn can lead to osmotic demyelination syndrome or other neurological complications [18]. It should also be noted that in our study we injected a bolus of 15% mannitol over 20-25 minutes. In a study by Battison et al, 2015, mannitol was administered as a 5 minute bolus dose. When mannitol was injected slowly (20-30 minutes), no ICP rebound syndrome was observed until 2 hours after infusion. In our studies, we also did not observe ICP "rebound" syndrome.
4. Conclusions
Therefore, it can be concluded that the rate of infusion may depend on the duration of the action of mannitol, because the faster the infusion, the greater the likelihood of termination of the effect due to rapid excretion of mannitol by the kidneys or its penetration into the brain tissue [19].Based on the whole of this brain, to say that mannitol (15%) reduces cerebral edema associated with TBI is a standard aid in the treatment of ICH. However, further research is needed to develop an ideal model of the pharmacokinetics of mannitol.
References
| [1] | Maas A.I.R, Menon D.K. et. al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. The lancet neurology commission| volume 16, issue 12, p987-1048, december 01, 2017. |
| [2] | Cnossen M.C., Huijben J.A. et. al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: a survey in 66 neurotrauma centers participating in the CENTER-TBI study. Critical Care volume 21, Article number: 233 (2017). |
| [3] | Adelson P.D., Bratton S.L. et. al. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatric Crit Car Med. 2003; 4: 40-44. |
| [4] | Kocheanek P.M., Carney N. et. al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012 Jan; 13 Suppl 1: S1-82. |
| [5] | Kochanek P.M., Carney N. et. al. Hyperosmolar therapy. J. Neurotrauma. 2007; 24(suppl 1): 14.20. |
| [6] | Hesdorffer D.C., Ghajar J. Marked Improvement in Adherence to Traumatic Brain Injury Guidelines in United States Trauma Centers. The Journal of Trauma: Injury, Infection, and Critical Care: October 2007 - Volume 63 - Issue 4 - p 841-848. |
| [7] | Tyagi R., Donaldson K., et. al. Hypertonic saline: a clinical review. J. Neurosurgical Review volume 30, pages 277–290 (2007). |
| [8] | Badjatia N., Carney N. et. al. Brain Trauma Foundation-2008. |
| [9] | Soni A., Chaudhary M., et. al. Impact of glycerol, mannitol, neurotol and neurotol plus administration in alcohol induced ischemic rat model. Trends in Medical Research 2009 Vol.4 No.3 pp.42-48 ref.27. |
| [10] | Sakellaridis N., Pavlou E. et. al. Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J. Neurosurg. 2011; 114: 545-548. |
| [11] | Cottenceau V., Masson F. et. al. Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. Journal of Neurotrauma 2011; 28: 2003-2012. tton. |
| [12] | Kinugasa K., M.D., Mandai Sh. et. al. Cellulose Acetate Polymer Thrombosis for the Emergency Treatment of Aneurysms: Angiographic Findings, Clinical Experience, and Histopathological Study. J. Neurosurgery, Volume 34, Issue 4, April 1994, Pages 694–701. |
| [13] | Luvisotto T.L., Roland N. et. al. The Effect of Mannitol on Experimental Cerebral Ischemia, Revisited. Neurosurgery, Volume 38, Issue 1, January 1996, Pages 131–139. |
| [14] | Turliuc M.D., Costachescu B., et. al. Rev. Med. Chir. Soc. Med. Nat. Iasi, 121, 533 (2017). |
| [15] | Dimitrios R. Panagiotis P. et. al. Effectiveness of 7.5% hypertonic saline in children with severe traumatic brain injury. Journal of Critical Care Volume 38, April 2017, Pages 52-56. |
| [16] | Manno E.M., Adams R.E., The effects of mannitol on cerebral edema after large hemispheric cerebral infarct. J. Neurology 52, 583. (1999). |
| [17] | Turliuc M.D., Cucu A.I., et. al. Efficiency and safety of microporous polysaccharide hemispheres from potato starch in brain surgerys. Cellulose Chem. Technol., 52 (7-8), 505-513 (2018). |
| [18] | Turliuc M.D., Cucu A.I., et. al. The use of mannitol in neurosurgery and neuro-ophthalmology. Cellulose Chem. Technol., 53 (7-8), 625-633 (2019). |
| [19] | Paczynski R.P. Osmotherapy: basic concepts and controversies. Critical Car Clinics. Volume 13, issue 1, p105-129, january 01, 1997. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML







