-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(11): 852-857
doi:10.5923/j.ajmms.20201011.05
Received: Oct. 17, 2020; Accepted: Oct. 29, 2020; Published: Oct. 30, 2020

Diagnostic Accuracy of the Soluble Fms-Like Tyrosine Kinase 1 / Placental Growth Factor Ratio and Polymorphism Agtr 1, Agtr 2 for Preeclampsia
Makhmudova Sevara Erkinovna1, Negmadjanov Bahodur Boltaevich2, Xamraev Xumoyun Xamzaevich1
1Department of Obstetrics and Gynecology, Faculty of Postgraduate Education, Samarkand State Medical Institute, Samarkand, Uzbekistan
2Department of Obstetrics and Gynecology №2, Samarkand State Medical Institute, Samarkand, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Preeclampsia (PE) is a disease that continues to be the main cause of maternal and fetal mortality and complications in 5-8% of pregnancies, Preeclampsia develops after 20 weeks of pregnancy and is characterized by hypertension and proteinuria. According to WHO, hypertension during pregnancy is the cause of 9 to 25 per cent of all maternal mortality, but accurate data are difficult to determine. In addition to the fact that PH is one of the leading causes of maternal and perinatal mortality, this disease and its complications cause a range of medical problems. Placental growth factor (PIGF) is relevant for healthy pregnancy, and abnormalities in PIGF functions have been associated with hypertensive disorders of pregnancy.
Keywords: Preeclampsia, Placental growth factor, Pregnancy, Proteinuria, Prognosis, Clinicо-genetic predictors, Polymorphism
Cite this paper: Makhmudova Sevara Erkinovna, Negmadjanov Bahodur Boltaevich, Xamraev Xumoyun Xamzaevich, Diagnostic Accuracy of the Soluble Fms-Like Tyrosine Kinase 1 / Placental Growth Factor Ratio and Polymorphism Agtr 1, Agtr 2 for Preeclampsia, American Journal of Medicine and Medical Sciences, Vol. 10 No. 11, 2020, pp. 852-857. doi: 10.5923/j.ajmms.20201011.05.
1. Introduction
- Preeclampsia is one of the most serious complications in obstetrics, which determines high rates of maternal morbidity and mortality. This pathology continues to be a dangerous complication of pregnancy and for the fetus, leading to delayed intrauterine development, premature birth, low birth weight and perinatal mortality. Preeclampsia was described as a “disease of theories” by Chesley in 1978 as the etiology of preeclampsia remained undefined. Recently, it has been believed that the pathogenesis of preeclampsia is associated with endothelium dysfunction, inflammatory response, oxidative stress, and dysfunction of renin-angiotensin system (RAS) [1]. Endothelial cells can regulate the contraction of underlying smooth muscle cells, prevent intravascular coagulation, and maintain the integrity of the intravascular compartment by producing a serials important substances such as nitric oxide (NO), endothelin-1, and tissue plasminogen activator [2], and modifying proliferation patterns of themselves. Some study suggested that proteinuria and cerebral edema present at early stage of preeclampsia might be ascribed to endothelial barrier damage [3]. So, the widespread endothelium dysfunction in matrix may be implicated in the development of preeclampsia. The cause of endothelium dysfunction has yet to be known. It is believed to be multifactorial, including disruption of cytokine function. There are many hypotheses for the occurrence of this pregnancy complication, among which the most relevant is the theory that considers preeclampsia as a multifactorial disease, where many genetic and environmental factors are involved in it's development. The ability to identify risk factors before pregnancy will allow timely assess the likelihood of developing preeclampsia and prescribe preventive treatment. To date, it has been established that more than 100 polymorphic variants of genes are associated with preeclampsia, in particular, genes of metabolism, the main complex of histocompatibility, lipid metabolism, cytokines and growth factors, hemostasis, regulation of endothelial function, vascular system, etc. However, differences in methodology for determining the severity of preeclampsia, the ethnicity of the surveyed, and combination of analyzed allelic variants in different samples determine the ambiguity of the results obtained by different authors. This dictates the necessity for in-depth research to identify the risk group, develop prognostic criteria, and conduct therapeutic and preventive measures to reduce perinatal losses and improve maternal and child health.It was found out that development of PE is based on a violation of placentation due to the defect in remodeling of myometrial vessels, which leads to incomplete invasion of trophoblast in the early stages of pregnancy. In future, the damaged ischemic placenta begins to secrete an excessive amount of a powerful antiangiogenic factor — a soluble receptor for vasculoendothelial growth factor (VEGF), identified as soluble fms-like tyrosine kinase 1 (sFlt-1). This factor inhibits both VEGF and placental growth factor (PIGF), which ensure normal placental development and function. Circulating in mother's bloodstream, sFlt-1 can contribute to the development of systemic endothelial dysfunction, which is the basis of all clinical manifestations of PE [4,5].There is reason to believe that the severity of clinical manifestations of PE is due to the period of pregnancy when it first started: the earlier PE debuts, the more severe it is [12].
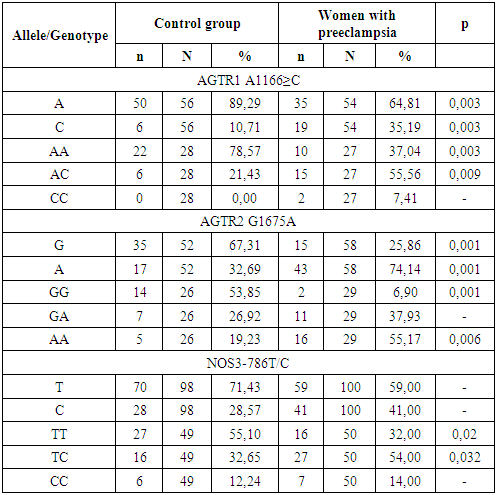
2. Materials and Methods
- We examined 160 pregnant women aged 18 to 40 years with a physiological course of pregnancy observed in the 2,3 –maternity complexes of Samarkand (11-40 weeks). The main and control groups were comparable in age, social characteristics, and obstetric and gynecological history. All women delivered healthy babies at term of 38-40 weeks, with rating on Apgar scale 8-9 scores, with normal weight and growth indicators. The postpartum period was uncomplicated for all of them. The exclusion criteria were multiple pregnancies, hypertension, and a history of preeclampsia. When assessing the reproductive function, it was found that the majority of women in both groups were primaparas (p ≥ 0.05). Pelvic inflammatory processes were equally common (p = 0.05). The group of patients with preeclampsia consisted of 82 pregnant women at 20-40 weeks, including 52 women with moderate preeclampsia and 30 with severe preeclampsia. The diagnosis of preeclampsia was established based on generally accepted criteria – hypertension (blood pressure ≥ 140/90 mmHg) and proteinuria (protein content above 0.3 g in daily urine). The severity of preeclampsia was assessed based on objective indicators and the patient's clinical condition. The group of patients with mild preeclampsia included pregnant women with blood pressure of 140-160/90 mmHg, with proteinuria of more than 0.3 g, but not less than 2 g/day. The group of patients with severe preeclampsia included pregnant women with blood pressure of 160/110 mmHg or more, with proteinuria of more than 2 g/day.The concentration of PIGF and sFlt-1 in the blood serum of pregnant women was determined using Elecsys PIGF and Elecsys sFlt-1 electrochemiluminescent diagnostic test systems of the Hoffmann La Roche concern (Switzerland) on a Cobas e411 automatic analyzer of the same company. The spectrum of the studied polymorphisms is presented in table 3. Total genomic DNA was isolated from 100 µl of whole venous blood by the sorbent method using the set "Proba-GS Genetics", NP-480-100 (AGTR1_1166 rs5186), NP-476-100 (AGTR2 G1675A rs1403543); for endothelial nitric oxide synthetase, 3 sets were used: NP-554-100 (eNOS_786 rs 2070744), NP-555-100 (eNOS_774 rs 1549758), NP-419-100 (eNOS_298 1799983); single-nucleoid polymorphisms were detected by real-time polymerase chain reaction using the above sets. (DNA technology, Russia).To obtain the serum, the samples were centrifuged for 15 min at 2000 rates and room temperature. The concentration of markers was determined on the same day, no later than 1.5 hours after blood collection. The concentration of PIGF and sFlt-1 in the blood serum of pregnant women was determined using Elecsys PIGF diagnostic test systems (Ref. no. 05144671190, Roche Diagnostics GmbH, Mannheim, Germany) and Elecsys sFlt-1 (Ref.no. 05109523190, Roche Diagnostics GmbH, Mannheim, Germany) on an automatic Cobas e411 electrochemiluminescence analyzer (Hitachi, Japan). The results were processed using the computer program Statistica.
3. Results
- The data obtained indicate that during the physiological course of pregnancy, the concentration of PIGF increases during 11-33 weeks of pregnancy and decreases sharply by the time of delivery. The concentration of sFlt-1 in healthy pregnant women begins to increase significantly from the 34th week of pregnancy and reaches its maximum values at 37-40 weeks of pregnancy, which is probably due to the necessity to reconstruct blood vessels in order to prevent massive bleeding during childbirth. The sFlt-1/PIGF ratio has maximum values in the period of 11-14 and 37-40 weeks of pregnancy, while the minimum values are observed in 24-33 weeks of gestation. (table 1)
|
|
|
|
4. Discussion
- Analysis of the obtained data indicates significant differences in the dynamics of PIGF and sFlt-1 concentrations and their ratio during physiological pregnancy and pregnancy complicated by preeclampsia. The most pronounced changes are in the values of the sFlt-1/PIGF ratio, in addition, the degree of deviation of the listed parameters correlates with the severity of preeclampsia. Apparently, this indicator is the most informative in the early diagnosis of preeclampsia. The results obtained are fully consistent with the published data that the development of preeclampsia is closely associated with an imbalance in synthesis of angiogenic and antiangiogenic factors [5,7,9]. It is known that the process of placenta formation begins with the implantation of cells of fetal origin (cytotrophoblast) into decidual tissue (modified endometrial layer of the pregnant uterus). The cytotrophoblast is not only embedded into endometrium layer (interstitial invasion) and spiral arteries (endovascular invasion), but also reaches the inner third of endometrium. As a result, at the end of the first trimester, several dozen wide, gaping arteries are formed in the utero-placental region and the utero-placental blood flow begins to function actively. Prior to its formation, the function of a powerful stimulus of the first wave of cytotrophoblastic invasion (CTI) is carried out by local tissue hypoxia, which is characteristic of the embryo microenvironment up to 8-10 weeks of development. Hypoxic stimulus increases the expression of specific cell adhesion molecules, stimulates the synthesis of cytokines and vascular growth factors [1].The presence of polymorphisms of genes that regulate vascular tone (renin-angiotensin system and endothelial nitric oxide synthetase) that predispose to hypertension complications significantly increases the risk of preeclampsia developing. The associations identified in this study can be used as genetic markers of predisposition to the formation of preeclampsia, which will allow timely formation of a risk group and correction of therapeutic and preventive measures.
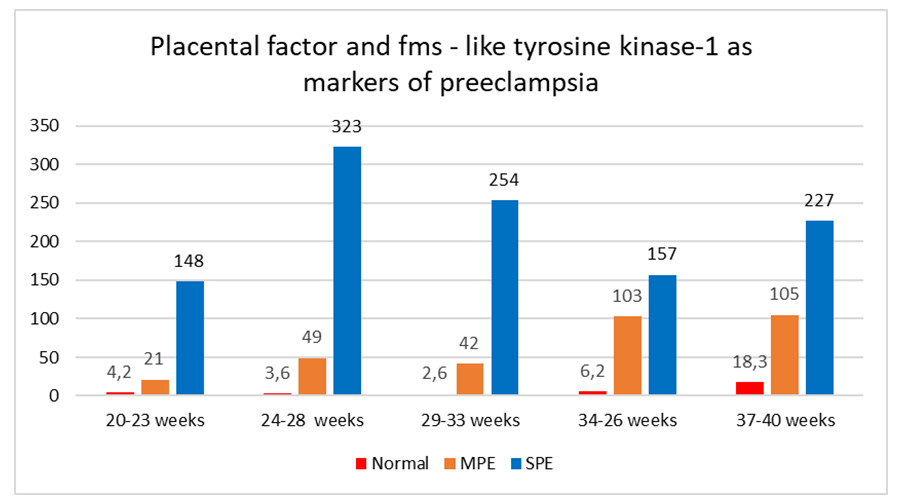 | Figure 1. The ratio of sFlt/PIGF in normal pregnancies, moderate (MPE) and severe preeclampsia (SPE) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML