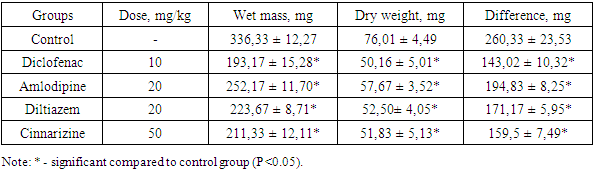-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(10): 817-821
doi:10.5923/j.ajmms.20201010.18
Received: Sep. 21, 2020; Accepted: Oct. 12, 2020; Published: Oct. 26, 2020

Specific Features of Exudative and Proliferative Phase of Inflammation When Using Calcium Channel Blockers
Z. Z. Khakimov1, A. Kh. Rakhmanov1, N. B. Bekova2, K. Sh. Shukurlaev2
1Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan
2Urgench Branch of the Tashkent Medical Academy, Uzbekistan
Correspondence to: A. Kh. Rakhmanov, Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of calcium channels blockers (CCB), such as diltiazem, amlodipine and cinnarizine was evaluated to explore the course of aseptic inflammation in sexually mature male rats. CCB in experimental animals show a remodeling effect on the exudative phase of inflammation, providing a distinct non- inferior anti exudative effect in large doses, compare to the classic nonsteroidal anti-inflammatory drug like diclofenac sodium. Interestingly, all investigated drugs suppress not only the exudative but also the proliferative phase of inflammation. Based on the results obtained, we recommended revising the structure of pharmacotherapy in patients taking into account comorbid diseases, the etiology of the inflammatory process of various localization.
Keywords: Calcium channels blockers, Anti-inflammatory effect, Inflammation
Cite this paper: Z. Z. Khakimov, A. Kh. Rakhmanov, N. B. Bekova, K. Sh. Shukurlaev, Specific Features of Exudative and Proliferative Phase of Inflammation When Using Calcium Channel Blockers, American Journal of Medicine and Medical Sciences, Vol. 10 No. 10, 2020, pp. 817-821. doi: 10.5923/j.ajmms.20201010.18.
Article Outline
1. Introduction
- Inflammation as a central link in the pathogenesis of many human pathologies is an urgent problem of modern medicine because, despite the introduction of a huge number of drugs of steroid and non-steroidal structures in the treatment of inflammatory diseases, especially chronic ones, there are still unresolved problems. This is largely due to the development of a number of side effects of medications [1,2,3,4], as well as the suppression of prostaglandin synthesis due to blockade of cyclooxygenase activity. In this regard, pharmacologists are faced with the task of creating new effective drugs with a different mechanism of anti-inflammatory action compare to the current ones. We have previously shown the anti-exudative effect of one of the representatives of calcium channel blockers (CCB) and cinnarizine [5]. However, in-depth studies of other drugs of this group could potentially be used for indications like non-steroidal anti-inflammatory drugs (NSAIDs), because calcium as an intracellular messenger plays an important role in the implementation of the functional activity of cells and its blockade would probably allow suppression of the inflammatory process. Proceeding from this, the purpose of this work was to study a number of CCB medications to investigate the course of aseptic inflammation.
2. Material and Methods
2.1. Experiments
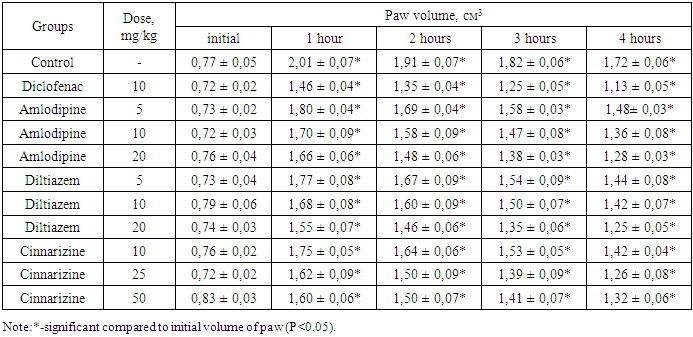
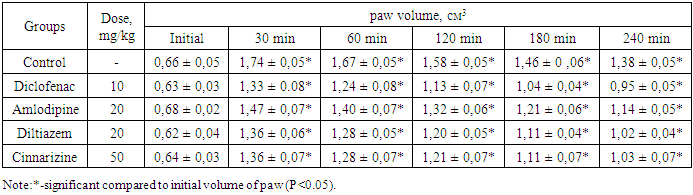
- All experimental studies were conducted on sexually mature male rats with an initial weight of 165-180 g. Animals were kept in standard vivarium conditions with free access to food and water, natural change of light and dark cycles, at room temperature 20-24°C. Animals were obtained from the vivarium of the Sanitary and Epidemiological Department of the Main Medical Directorate under the Administration of the President of the Republic of Uzbekistan. The following preparations were used in the experiments such as diclofenac sodium (JSC "Sintez", Russia); diltiazem Lannacher (Lannacher Heilmittel GmbH, Austria), amlodipine (RUE Belmedpreparaty, Belarus), cinnarizine (Experimental Plant GNTSLS, Ukraine). Experimental studies were carried out in accordance with the "Rules for laboratory work using experimental animals", as well as the rules given in the European Convention for the Protection of Vertebrate Animals used for Experimental Research or for Other Scientific Purposes (ЕТS No. 123) Strasbourg, 03/18/1986. Each group of the study contained 6 animals. The anti-exudative effect of the drugs was studied on a model of acute inflammatory edema of the rat paw induced by the introduction of 0.1 ml of 6% dextran solution and 0.1% histamine solution under the plantar aponeurosis of the right hind limb of the animal [6,7]. Diclofenac sodium at a dose of 10 mg/kg was administered into the stomach by metallic cannula to experimental animals 1 hour prior the induction of the inflammation; amlodipine and diltiazem at doses of 5, 10, and 20 mg/kg, cinnarizine at doses of 10, 25 and 50 mg/kg, respectively. The control group included rats received an equal volume of distilled water. The volume of the right hind paw of the animals was measured using a plethysmometer at the baseline before any manipulations, and 1, 2, 3, and 4 hours after the injection of the flagogen. Based on the data of the average increase in the volume of the paws of animals, obtained as a result of parallel measurements, the values of anti-inflammatory activity (AIA) in % were calculated using the formula:
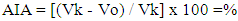 Where Vk is the average increase in the volume of the limb in the control, Vо is the average increase in the volume of the limb in the experiment [5]. If the PVA value exceeds 30%, then, as is commonly believed, the drug has a pronounced anti-inflammatory effect [8].The investigated drugs were also studied for the proliferative phase of inflammation on the model of "cotton granuloma" [9,10]. This model was created by implanting a sterile cotton swab (10 mg of weight) in rats under the skin of the back between the scapulas. The operation was performed under aseptic conditions using general anesthesia. Injection of the medications into the stomach was carried out once daily before meals, the day of the operation, and every day next seven days in the following doses: Diclofenac sodium 10 mg/kg, amlodipine, and diltiazem in a dose of 20 mg/kg each, and cinnarizine 50 mg/kg. Control animals received distilled water in the comparable volume. 24 hours after the last administration of the drugs (on the eighth day), the animals were sacrificed under general anesthesia, and cotton balls with the granulation tissue formed around them were removed, weighed on an electronic balance (SINKO, Japan, 2014), and dried at 60°C to constant weight. The degree of the proliferative phase was estimated by the difference between the mass of the dried granuloma and the initial mass of the ball. The exudative reaction was assessed by the difference between the weights of the raw and dried granulomas [11,12].
Where Vk is the average increase in the volume of the limb in the control, Vо is the average increase in the volume of the limb in the experiment [5]. If the PVA value exceeds 30%, then, as is commonly believed, the drug has a pronounced anti-inflammatory effect [8].The investigated drugs were also studied for the proliferative phase of inflammation on the model of "cotton granuloma" [9,10]. This model was created by implanting a sterile cotton swab (10 mg of weight) in rats under the skin of the back between the scapulas. The operation was performed under aseptic conditions using general anesthesia. Injection of the medications into the stomach was carried out once daily before meals, the day of the operation, and every day next seven days in the following doses: Diclofenac sodium 10 mg/kg, amlodipine, and diltiazem in a dose of 20 mg/kg each, and cinnarizine 50 mg/kg. Control animals received distilled water in the comparable volume. 24 hours after the last administration of the drugs (on the eighth day), the animals were sacrificed under general anesthesia, and cotton balls with the granulation tissue formed around them were removed, weighed on an electronic balance (SINKO, Japan, 2014), and dried at 60°C to constant weight. The degree of the proliferative phase was estimated by the difference between the mass of the dried granuloma and the initial mass of the ball. The exudative reaction was assessed by the difference between the weights of the raw and dried granulomas [11,12].2.2. Statistical Analysis
- The standard StatPlus 2009 software package and the method of variation statistics was used. Continuous data is presented as (M ± m), and the statistical differences between groups was calculated using Student's t-test. The difference was considered significant at a probability level of 95% or more (p <0.05).
3. Results and Discussion
- The results of the experiments showed that CCB medications had a definite remodeling effect on the exudative process of inflammation. Thus, the control group of rats after supplantation of dextran the paw volume increases after 1 hour by 161.0%, after 2 hours - by 148.1%, after 3 hours - by 136.4%, and after 4 hours by 123.3% compared to the baseline data. In rats receiving amlodipine at a dose of 5 mg/kg, there were 146.5, 131.5, 116.4, and 102.7% increase, respectively. Under the influence of amlodipine, the increase in the paw volume of rats was less significant compare to controls. At the same time, the calculation of AIA in the indicated study periods was 13.7, 15.8, 19.0, and 21.0%, respectively. As can be seen from the data in Table 1, an increase in the dose of the drug by two and four times led to an increase in the noted effect, especially at a dose of 20 mg/kg.Consequently, the assumption that vasodilation and a decrease in blood pressure should intensify the exudation process was not confirmed in our experiments. Moreover, with an increase in the dose of the drug, the degree of hypotension should probably increase, however, the exudation process, as can be seen from the data presented, on the contrary, decreases. It was necessary to establish whether this effect was associated with the pharmacological properties of amlodipine particularly, or this is specific to all calcium channel blockers. To explore this, under similar experimental conditions, we conducted a study to examine the effect of another CCB diltiazem. As can be seen from the data in tables 1, the action of diltiazem was similar to amlodipine but slightly more pronounced. If at the peak of exudation the AIA of amlodipine at a dose of 20 mg/kg was 27.4 - 45.3%, then for diltiazem at a similar dose was 34.7 - 46.3%.The CCB amlodipine, diltiazem has a distinct anti-exudative effect. It is known that these drugs may have general anti-spasmatic effects by lowering the tone of the cerebral vessels, enhancing cerebral circulation. Cinnarizine is one of the drugs that have a relatively selective cerebrovascular effect, as a result of which not only cerebral but also coronary circulation improves [13,14]. It is believed that the drug, along with the fact that it reduces the response to biogenic vasoconstrictor substances (such as angiotensin), also has antihistaminic activity [13]. It is known that histamine is one of the important mediators of inflammation [15], based on this, it can be assumed that cinnarizine may have an anti-exudative effect. The results of experimental studies in animals preemptively receiving cinnarizine, the degree of the exudation process decreased. As can be seen from the data in Table 1, almost identical results were obtained by us in animals that were previously administered with cinnarizine. Thus, in rats previously treated with cinnarizine at a dose of 10 mg/kg, the paw volume increased by 130.2, 115.8, 101.3, and 86.8%, respectively, after 1, 2, 3, and 4 hours from the beginning of the experiment. Under the influence of the cinnarizine at a dose of 25 and 50 mg/kg, the paw volume increased by 125.0 and 92.8% after 1 hour, 108.3, and 80.7% after 2 hours, and by 93.1% after 3 hours, and 69.9% and after 4 hours by 75.0 and 59.0%. The values of anti-inflammatory activity were 37.0 - 44.0% (at a dose of 25 mg / kg) and 37.9 - 48.4% (at a dose of 50 mg / kg), respectively, after 1 - 4 hours of observation.
|
|
|
4. Conclusions
- 1. Calcium channel blockers such as cinnarizine, diltiazem, and amlodipine in experimental animals have a modeling effect on the exudative phase of inflammation, providing a distinct anti-exudative effect in higher doses, compare to the classic nonsteroidal anti-inflammatory medication like diclofenac sodium.2. The investigated drugs suppress not only the exudative but also the proliferative phase of inflammation.3. The mechanism of the anti-inflammatory action of calcium channel blockers is probably associated with a decrease in the release of inflammatory mediators in connection with the loss of the function of calcium ions acting as a secondary messenger.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML