-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(10): 741-745
doi:10.5923/j.ajmms.20201010.03
Received: Jul. 28, 2020; Accepted: Sep. 3, 2020; Published: Sep. 26, 2020

Effects of African Walnut (Tetracarpidium Conophorum) on Some Parameters of Kidney Function in Male Albino Rats
Eneh F. U.1, Eneh C. I.2, Eziewe C. M.1, Agbata C. P.1
1Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
2Department of Paediatrics Enugu State University Teaching Hospital, Enugu, Enugu State, Nigeria
Correspondence to: Eneh F. U., Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effects of African Walnut Tetracarpidium conophorum on some parameters of kidney function were investigated on male albino rats. A total of fifteen rats were used for the investigation. There were 3 rats in the baseline group while the control, test 1 and test 2 groups had four rats each. The baseline was sacrificed on the day the animals were purchased to determine the initial values of the parameters. The control was fed commercial animal feed (grower’s mash) while the test 1 and test 2 groups were fed the commercial feed with 2.50% and 5.00% walnut flour respectively. After 21 days of feeding, the rats were sacrificed and the renal parameters were determined. The weights of the animals in the different groups were measured at specific time intervals. The serum urea concentration for the baseline control, test 1 2.5% and test 2 5.0% groups were 28.20 ± 2.20 mg/dl 25.20 ± 4.39, 5.40 ± 2.00 and 23.86 ± 2.90 respectively. The walnut at 2.5% inclusion rate was able to significantly (P<0.05) reduce the serum urea concentration in comparison to the baseline, control and test (2) 5.0% groups. The serum creatinine concentration for the baseline, control, test 1 and test 2 groups were 4.83 ± 3.38 mg/dl 0.91 ± 0.32, 1.05 ± 0.29 and 3.08 ± 0.72 respectively. The control and the two test groups had lower creatinine values than the baseline. The difference between the control and the test 2 group was statistically (P<0.05) significant while the test 1 group attained almost the same value as the control and so the difference was not statistically significant. This shows that walnut at low inclusion ratio produced a normal serum creatinine level and was not harmful to the kidney. The mean percentage changes in weight for the control test 1 and 2 groups were 9.44, 2.83 and 2.75 respectively showing that walnut suppressed growth in a concentration dependent manner. These findings show that walnut has a great potential for the management of uraemia without adversely affecting renal clearance.
Keywords: Tetracarpidium conophorum, Urea, Creatinine, Uraemia, Clearance, Kidney
Cite this paper: Eneh F. U., Eneh C. I., Eziewe C. M., Agbata C. P., Effects of African Walnut (Tetracarpidium Conophorum) on Some Parameters of Kidney Function in Male Albino Rats, American Journal of Medicine and Medical Sciences, Vol. 10 No. 10, 2020, pp. 741-745. doi: 10.5923/j.ajmms.20201010.03.
Article Outline
1. Introduction
- There is an increase in the number of kidney diseases with debilitating consequences despite the medical and surgical interventions thereby necessitating supportive approaches like nutritional interventions and lifestyle modifications as options that could assist to control the rise. kidney diseases cause harm or damage to the kidneys with the attendant deterioration of the body's ability to purify the blood, eliminate excess water in the blood, maintain electrolyte balance and control blood pressure. Acute kidney diseases have sudden onset while the chronic types have persisted over a long period of time. These diseases have different clinical manifestations and include among others glomerulonephritis, pyelonephritis, nephrotic syndrome, acute and chronic renal failures, polycystic kidney disease, urinary tract infections, and kidney stone (Davison et al 1998, Nsirim, 1999).The causes include bacterial infections, some plant extracts (oxalates), tubular nerosis circulatory insufficiency, (Nsirim 1999) renal poisons like heavy metals, carbontetrachloride, unregulated use of drugs like non-steroidal anti-inflammatory drugs; congentital factors and excess sodium among others. The risk factors are high blood pressure (Boon and Fox 1998), diabetes mellitus Type I and II (Edwards et al 1998), family, heredity, history, age, and race. It is estimated that about 26million Americans suffer from kidney diseases while in Nigeria about 100,000 new cases are occur yearly. Among the risk factors diabetes mellitus alone accounts for 44% of the new cases. Several biomarker exist for the assessment of renal function however, the common ones include serum creatinine, blood urea nitrogen (BUN), urinary albumin/protein and volume of excretion. Assessment also includes ultrasound or computed tomography (CT) scan and renal biopsy.Control and treatment options include regulation of high blood pressure, blood glucose and cholesterol levels, reduced alcohol intake, quiting of smoking, loss of weight, increased physical activity, dialysis, renal transplant, healthy fruits, and vegetable intake. It is in the light of management or control of renal diseases from the perspective of plants and vegetables that the engagement of African Walnut (Tetracarpidium conophorum) for this purpose comes into focus. It is a tropical rambling perennial woody plant of the family Euphorbiaceae and is widely distributed and consumed by the inhabitants of Africa (Kanu et al 2015). African walnut is rich in proteins, carbohydrates, lipids, vitamins, and minerals (Onawumi et al 2013). The oil contains omega -3 fatty acids and has cholesterol lowering potential. The plant has many phytochemicals which include tannins, cyanides, terpenoids, saponins, steroids, cardiac glycosides, flavonoids, and alkaloids (Igara et al., 2017). African walnut has been reported to have male fertility activity (Obianime and Uche 2010), hypoglycaemic activity (Onwuli et al., 2014) and antiulcer activity, (Ezealisiji et al 2014). The boiled nut is commonly consumed and enjoyed as a snack and in view of the several reported health benefits and the rising cases of renal disease and the associated risk factors it is worthy to investigate if African walnut would ameliorate renal conditions.
2. Materials and Methods
- MATERIALS:Walnut samples were purchased at Ose Market Onitsha Anambra State Nigeria and identified at the Botany Laboratory Nnamdi Azikiwe University Awka. The Male albino rats were purchased at Anagonye farms Awka. The creatinine and urea kits were produced by Randox (USA).METHODS:PROCESSING OF WALNUT: The walnut samples were air dried and boiled in water. The boiled samples were milled and dried in the oven at 50°C. The oven dried sample was then stored in a plastic container. GROUPING OF ANIMALS AND FEEDING:The animals were separated into four groups; Group 1 (baseline) had 3 rats while Group 2 (control), Group 3 (test 1) and Group 4 (test 2) had four rats each. The baseline group was sacrificed on the first day the rats were purchased to determine the initial values of the parameters being assayed for. The control group was fed with a commercial feed only while the test groups 1 and 2 received the commercial feed mixed with the processed walnut at inclusion rates of 2.5 and 5.0% respectively for a period of 21 days.Sacrifice of Animals and Blood collection:The rats were anaesthesized with cotton wool soaked in chloroform. The blood was collected through cardiac puncture and transferred to universal bottles and allowed to clot after which they were centrifriged for 10 minutes at 4000rpm. The sera obtained were transferred into test tubes and used for biochemical analysis. Biochemical Analysis: Determination of Urea Concentration.The serum urea concentration was determined with Randox kits based on the method of Fawcett and Scott (1961). The method is based on the reaction of urea and water in the presence of urease Urea + H20 = 2NH3 + Co2.Salicylate and hypochlorite in the reagent react with ammonium ions to from a green coloured complex (2, 2 dicarboxylindophenol) and the absorbance read at 546nm. The amount of the coloured complex formed is proportional to urea concentration. The blank, standard, and sample (10micro litere each) were pipelted into test tubes and 100 microlitre of sodium nitoprusside and urease were added to the tubes. The contents of the tubes were mixed at 37°C for 10 minutes. Phenol concentrate and hypochlorite were added to the tubes and they were mixed and incubated at 37°C for 10 minutes. Absorbance of the samples and standard were read against a blank at 546nm. Urea concentration =
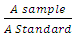 x Concentration of standard (mg/dl)A sample = absorbance of sampleA standard = absorbance of standard Determination of creatinine concentration.Creatinine in alkaline solution reacts with picric acid to form coloured complex and the amount formed is directly proportional to the concentration of creatinine. The creatinine concentration was determined with the Randox kit based on the colorimetric method of Bartels and Bohmer (1972). The working reagent (1.0ml) was added to standard solution (0.1ml) and serum sample (0.1ml) seperately in different test tubes. After 30 seconds, the absorbance A1 of the standard and sample were read at 492nm. Exactly 2 minutes later the absorbance A2 of the standard and sample were read at 492nm.Creatinine content calculation.Creatinine concentration =
x Concentration of standard (mg/dl)A sample = absorbance of sampleA standard = absorbance of standard Determination of creatinine concentration.Creatinine in alkaline solution reacts with picric acid to form coloured complex and the amount formed is directly proportional to the concentration of creatinine. The creatinine concentration was determined with the Randox kit based on the colorimetric method of Bartels and Bohmer (1972). The working reagent (1.0ml) was added to standard solution (0.1ml) and serum sample (0.1ml) seperately in different test tubes. After 30 seconds, the absorbance A1 of the standard and sample were read at 492nm. Exactly 2 minutes later the absorbance A2 of the standard and sample were read at 492nm.Creatinine content calculation.Creatinine concentration =  x Concentration of standard (mg/dl)A1 (sample) = initial absorbance of sampleA2 (sample) = final absorbance of sample A1 (standard) = initial absorbance of standardA2 (standard) = final absorbance of standard.Measurement of Weights of RatsThe weights of the animals were taken on the day, 1 2, 7, 14 and 21. The group means were calculated. The plot of the mean percentage differences in weight over time (days) from the first day for the control, 2.5 and 5.0% groups was constructed. Statistical Analysis T-tests (paired comparisons) were used to compare initial and final weights in each of the groups.T-tests (assuming equal variances) were used to compare initial and final weights. ANOVA was used to compare all the parameters between and within the treatment groups to check for significant differences. Values were considered significant at p <0.05.
x Concentration of standard (mg/dl)A1 (sample) = initial absorbance of sampleA2 (sample) = final absorbance of sample A1 (standard) = initial absorbance of standardA2 (standard) = final absorbance of standard.Measurement of Weights of RatsThe weights of the animals were taken on the day, 1 2, 7, 14 and 21. The group means were calculated. The plot of the mean percentage differences in weight over time (days) from the first day for the control, 2.5 and 5.0% groups was constructed. Statistical Analysis T-tests (paired comparisons) were used to compare initial and final weights in each of the groups.T-tests (assuming equal variances) were used to compare initial and final weights. ANOVA was used to compare all the parameters between and within the treatment groups to check for significant differences. Values were considered significant at p <0.05.3. Results
- The results of the mean changes in weights (expressed as mean percentage change in weights), mean urea, and creatinine are shown in figure 1 and tables 1 and 2.
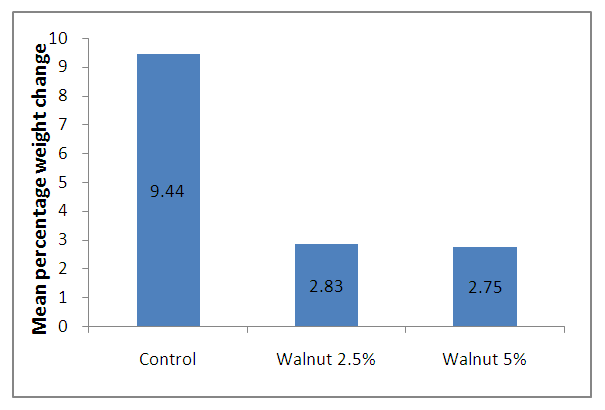 | Figure 1. Mean Percentage Weight Changes for the Control, Test 1 and Test 2 |
|
|
4. Discussion
- Consumption of walnut by rats in this study did not produced appreciable changes in their weights. After the feeding period of 21 days the control, test 1 and 2 groups attained the mean percentage change (increase) in weight of 9.44, 2.83 and 2.75 respectively. This suggests that walnut has suppressive effect on growth in a dose dependent manner since higher concentration of walnut produced lower percentage change in weight and vice versa. The implication is that further reduction in the concentration of walnut could eliminate the suppression of growth while increasing the concentration of the walnut will enhance the suppressive effect. The implication is that walnut could be beneficial in conditions where suppression of growth or proliferative tendencies is required such as in the cancerous state or included sufficiently in dietary formulations for weight reduction therapy The control however, without walnut had a much higher percentage change in weight than the two test groups. However, the differences in mean percentage changes in weight among the three groups were not statistically (p>0.05) significant.Chronic kidney disease (CKD) has been shown to be caused by many factors such as diabetes, High blood pressure, kidney stones, glomerulonelities, polycystic kidney disease s also one of the major causes of fertility among patients with CKD. As a result of CKD, the kidney may begin to compromise its ability to eliminate urea. Thus CKD is a risk factor for uraemia. Uraemia toxins could therefore be the underlying factors for the pathogenesis of CVD and CKD (Tonelli et al, 2016). Uraemia may ultimately cause kidney failure for which patients will be left with the options of dialysis or kidney transplant. Walnut consumption was able to cause a fall in the serum urea level. The baseline group had a mean serum urea concentration of 28.20 ± 2.20mg/dl while the control group had the mean value of 25.20 ± 4.39. The test groups of 2.5 and 5.0% walnut intake had values of 5.40 ± 2.00 and 23.86 ± 2.90.So at a 2.5% inclusion rate in test (1) group the walnut was able to significantly reduce (P<0.05) the serum urea level when compared to the baseline, control and test (2) groups. The differene in urea concentration between the two test groups is statistically (p<0.01) significant. However, the difference between baseline and control urea concentration was not statistically (p>0.05) S significant. Walnut could therefore be beneficial to CKD patients suffering from ureamia because of its ability to reduce serum urea concentration. Ethenol extract of ginger has been shown to markedly decrease blood urea nitrogen and is therefore considered beneficial in the management of patients with ureamia (Mehrdred et al, 2007). Plants used in the treatment of kidney failure were reported by Yarnell and Abascal (2007).Walnut consumption reduced the creatinine level when compared to the baseline values. The baseline value was 4. 83 ± 3.38 mg/dl while 5.0% test group had a value of 3.08 ± 0.72 which had no statistically significant (p>0.05) difference from the baseline. The creatinine level for the control group reduced to a value of 0.91 ± 0.32 which was statistically significant (p<0.05) when compared to the 5% test group. However, the 2.5% test group reduced the serum creatinine concentration to a value of 1.05 ± 0.29 which is close to the value attained by the control. At the test inclusion ratios of 2.5 and 5.0% walnut gave serum creatinine values lower than the baseline and at 2.5% inclusion ratio, it produced a serum creatinine concentration of almost the same value as the control without any statistically significant difference (p>0.05) thereby indicating that walnut has no nephotoxic effects at these inclusion ratios. Comparison of the creatinine values of the two test groups showed a statistically (p= 0.02) signiticant difference.Plant extracts are very promising as sources of remedies for the treatment of renal diseases. In doxorubicin induced nephrotoxicity there were elevations of serum urea, uric acid and creatinine and reduction of albumin indicating poor clearance by the kidney. However, in the animal group treated with Costus pitus plant extract serum concentrations of albumin, urea, uric acid and creatinine were not significantly affected showing that herbal extract of Cotus pitus offered apprreciable protection to the kidney (Rejasekaran, 2019). Also rats treated with extracts of Vernonia calvoana leaves experienced a reduction in serum urea and creatimine concentrations in renal challenge induced by acetaminophen indicating the potential renoprotective effect of the plant (Egbung et al, 2017).
5. Conclusions
- African Walnut has proved to be beneficial in the management of uraemia. At 2.5% inclusion rate it reduced the serum creatinine concentration more than the baseline and was comparable or almost the same value as the control indicating no hazardous effect. At 5.0% inclusion ratio the serum creatinine levels increased. This increment might have elevated the concentration of photochemical like oxalates among others which reduced the creatinine clearance by the kidney. Walnut contains oxalates (Ojobor et al 2015) which form calcium oxalate crystals that constitute kidney stones and affect renal functions. Processing of walnut like prolonged boiling, heating or roasting could reduced the levels of the photochemical (except where reduction of clearance is not necessary) in such a way that there will be an enhancement of renal clearance without adversely affecting its ability to control uraemia. However, at 2.5% inclusion rate walnut appears to be generally beneficial to the renal system. Nutritional intervention through walnut on kidney challenges should be encouraged.
Ethical Approval
- The animals were handled following standard ethical guidelines on animal handling and research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML