Z. I. Ubaidullaeva, H. R. Tursunova
Scientific and Production Enterprise "Blood Products", General Department of Health of Tashkent City, Republic of Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The features of the enzyme system of red blood cells during long-term storage were studied. 80 doses of erythrocyte mass of various storage periods were examined. In erythrocytes washed with physiological solution (pH 7.2), the following values were determined: methemoglobin content, hexokinase activity, glucose-b-phosphate dehydrogenase, glutathione reductase, catalase and K +, Na + dependent ATPase of erythrocytes. The traditional storage of erythrocyte mass for 30 days has been revealed, accompanied by the development of oxidative stress and hemolytic processes in the cells, which lead to a violation of the morphofunctional usefulness and viability of blood cells by 7-14 days of storage.
Keywords:
Erythrocyte mass, Enzymes of carbohydrate metabolism, Functional state of red blood cells
Cite this paper: Z. I. Ubaidullaeva, H. R. Tursunova, Influence of Erythrocyte Mass Conservation Duration on Indices of Enzyme Systems of Carbohydrate Exchange, American Journal of Medicine and Medical Sciences, Vol. 10 No. 10, 2020, pp. 733-735. doi: 10.5923/j.ajmms.20201010.01.
1. Введение
According to domestic and foreign studies, at present there is a tendency to increase the use of blood and its preparations in practical medicine, in particular, using of erythrocytic mass [2,5,6]. The latter becomes the main means of transfusion treatment of acute and chronic anemic conditions. The erythrocytic mass is the main cell substrate approximating to whole blood in its composition, functional properties and therapeutic efficiency [7,8]. However, in the erythrocyte mass, as in whole blood, significant changes occur during the preservation process. Storage of red blood cells at + 4°C adversely affects the course of metabolic processes in red blood cells. Also, during storage, conditions that contribute to the activation of lipid peroxidation processes are created. In a normally functioning cell, this process is effectively suppressed by the antioxidant defense system, in which catalase plays the main role [9,10,11]. However, progressive acidification of the blood due to the accumulation of lactate during storage has an inhibitory effect on the enzyme. Low pH also adversely affects the activity of key glycolysis enzymes and the pentose phosphate cycle. At the same time, the ability of red blood cells to utilize glucose is reduced. Due to the fact that the enzymes of glucose metabolism are associated with the membrane, the intensification of lipid peroxidation (lipid peroxidation) and a change, as a result of this, in the lipid environment of glycolytic enzymes affect their activity. These same factors, together with a decrease in ATP concentration due to a decrease in metabolic activity, affect the activity of ion transport enzymes, primarily K, Na + -ATPase [12,13,14]. These changes can not only reduce the effectiveness of blood transfusions with red blood cells, but also contribute to the development of post-transfusion complications associated primarily with disorders of microcirculation in the lungs, kidneys, and liver. The purpose of this research work was to study the effect of different retention times of canned red blood cell mass on the biochemical properties of red blood cells.
2. Material and Research Methods
We analyzed 80 doses of washed red blood cells with different storage periods. The main object of research is red blood cells of donated blood. In erythrocytes washed with physiological solution (pH 7.2), the following values were determined: methemoglobin content (F.I. Ataullakhanov et al., 1984), the level of free plasma hemoglobin was determined by V.V. Menshikov, (1987), hexokinase and glucose activity β-phosphate dehydrogenase glutathione reductase (Kochetov G.A., 1980). Antioxidant activity was judged by catalase. The activity of erythrocyte catalase (CT, µkat/l) was determined by the method of M.A. Korolyuk (1988).The results were statistically processed using the Statistica 6.1 application package. The Student's t-criterion was used to evaluate the reliability of differences between groups. Spearman's correlation analysis was used. The critical value of p significance level was assumed to be 0.05.
3. Research Results and Discussion
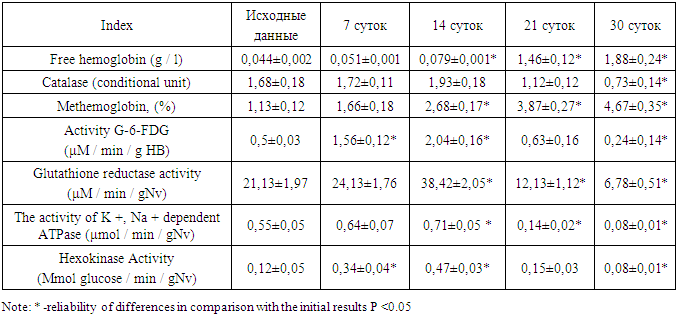
In a study of erythrocytic mass obtained from donors, an inverse relationship was found between the level of hemoglobin and the duration of storage - the longer the storage time, the less hemoglobin in the dose. At the same time, the level of free hemoglobin, on the contrary, increased as the duration of storage of red blood cells increased (Table 1). So, after 14 days, the free hemoglobin level increased 1.8 times compared to the initial indicators. After 30 days, the concentration of free hemoglobin reached its maximum values and exceeded the initial results by 43 times. High values of free hemoglobin in the erythrocyte mass can adversely affect the functional state of platelets and enhance its adhesive-aggregation state (V.V. Lebedev, 2013). Therefore, storage of erythrocytes for more than 30 days leads to changes in their quality characteristics, which is manifested by an increased degree of erythrocyte hemolysis and a decrease in the amount of hemoglobin in the dose.Table 1. Dynamics of indicators of enzymatic systems of carbohydrate metabolism of red blood cells during storage
 |
| |
|
The progression of LPO in late storage is likely due to an increasing deficiency of antioxidant defense factors. Thus, in erythrocyte mass on the 21st and 30th days of storage a decrease in catalase activity by 33% and by 57% relative to the initial parameters was observed. In erythrocytes it increased to 14 days and then decreased. This condition can also be caused by changes in morphological characteristics of cells during erythrocyte storage. At the same time, cells with worse morphological characteristics are destroyed.The study of erythrocyte condition indicators reveals changes that testify to the appearance of high rate of methemoglobinogenesis in the cell population (Table 1). The growth of methglobin formation rate in erythrocytes along with the insufficiency of their reduction systems can be accompanied by increased fixation of MetHb in the structural components of the membrane, forming a membrane-bound hemoglobin (MbHb), which changes the structural and functional state of erythrocytes.It is known that the non-enzymatic oxidation of hemoglobin (Fe2+) in methemoglobin (Fe3+) leads to one-electronic oxygen recovery and the appearance of reactively capable anion radical - superoxide O2~, which is a precursor of other active forms of oxygen peroxide: hydrogen peroxide H2O2 and hydroxyl radical OH'. In erythrocytes, as in most cells, there is a thiol-containing tripeptide - glutathione (corneramyl-cystenyl-glycine). The reduced form of glutathione (G-SH) contains an SH-group, which can serve as an electron donor in reduction reactions. Under the action of the enzyme glutathione peroxidase reduced glutathione converts a molecule of hydrogen peroxide into a molecule of water, and itself goes into an oxidized state (G-SS-T). Regeneration of the reduced glutathione is ensured by glutathione reductase, using hydrogen hydrogenated NADPH as a donor. For erythrocytes, the only source of NADPH is the pentosophosphate pathway. The interaction of reduced glutathione with hydrogen peroxide in erythrocytes protects cysteine residues in hemoglobin protomers from oxidation. In our studies, glutathione reductase levels rise to 14 days and then fall sharply. Similar dynamics have been observed with respect to glucose-6-phosphate-degrogenase in erythrocytes. At low activity of glucose-6-phosphate dehydrogenase the concentration of reduced coenzyme NADPH decreases, resulting in a sharp decrease in the concentration of reduced glutathione, and in the cell, respectively, increases the number of active oxygen forms. In this case, the oxidation of SH-groups of hemoglobin molecules in red blood cells leads to the formation of cross-disulfide bonds and aggregation of hemoglobin protomers with the formation of Heinz cells. In the presence of Heinz cells, the plasticity of the membrane is impaired and the membrane loses its ability to deform when erythrocytes pass through the capillaries and causes damage to the integrity of erythrocyte membranes.As can be seen from the presented research results, the activity of regulatory enzyme glycolysis in erythrocytes increases reliably up to 14 days of storage of erythrocyte mass, then decreases sharply, thus indicating a decrease in energy production in the form of ATP. A low level of macroerg in red blood cells affects the membrane enzyme K +, Na + dependent ATP-zy, the dynamics of which is directly dependent on the activity of hexokinase and indicates morphological changes in red blood cells. The obtained results allow to consider changes in carbohydrate metabolism indices in red blood cells as a result of changes in intracellular pH and temperature. Deterioration of erythrocyte mass quality when stored for a long period of time can be mediated by an increase in the level of active oxygen forms due to a decrease in the activity of erythrocyte antioxidant enzymes. It can be assumed that under the influence of POL products erythrocyte membrane damage occurs.
4. Conclusions
1. The traditional storage of red blood cells for 30 days is accompanied by the development of oxidative stress and hemolytic processes in the cells.2. Identified violations in the enzyme system of red blood cells lead to a violation of the morphofunctional usefulness and viability of blood cells by 7-14 days of storage.
References
| [1] | Ataullakhanov F.I., Vitvitsky V.M., Zhabotinsky A.M., Pichugin A.V., Pomazanov V.V., Titkova N.F. The effect of glycolysis on the metabolism of adenylates in human red blood cells. // Biochemistry. 1984a. T. 49. No. 1. S. 104-110. |
| [2] | Byakin S.P., Makedonskaya O.G., Piksin I.N., Averina A.V., Makhrov V.I. To the question of the clinical and economic efficiency of the use of certain transfusiological methods of treatment in medicine // Problems of experimental and clinical surgery: Sat. scientific labor. - Saransk: IMU, 2014 .-- S. 27-30. |
| [3] | Korolyuk MA, Ivanov LI, Mayrova I.G. et al. Method for determining catalase activity. Lab affair 1988; 51: 16-9. |
| [4] | Kochetov G. A. A Practical Guide to Enzymology / G. A. Kochetov. - M.: Higher School, 1980. - 271 p. |
| [5] | Krasnyakov V.K. Improving blood donation and its components in St. Petersburg / Diss. . Cand. honey. Sciences. - SPb., 2010. 122 p. |
| [6] | Zhiburt E.B. Transfusion media / E.B. Zhiburt // In the book: Transfusiology. - St. Petersburg: Peter, 2004. -- S. 323-369. |
| [7] | Lebedev V.V., Moisova D.L., Podsadnaya A.A., Svistunov N.V. Hemolytic-uremic syndrome as a variant of the pathology of the hemostasis system in case of Lertospirosis // Basic research. - 2013. - No. 7-2. - S. 334-338. |
| [8] | Mazova A.B. Immunohematological and infectious blood parameters of donors and patients during blood transfusion therapy in pediatric practice / Diss. . Cand. honey. Sciences .- M., 2008. - 118 p. |
| [9] | Makedonskaya OG, Byakin SP, Zorkina AV, Fedoseykin IV, Baytyakov VV Lipid peroxidation and antioxidant protection of erythrocytes during bubbling aeroionization of donor erythrocyte mass // Human Physiology. - 2010. - T. 36, No. 1. - S. 142-144. |
| [10] | Menshikov V.V., Delektorskaya L.N., Zolotnitskaya R.P. and etc.; Edited by V.V. Menshikov. Laboratory research in the clinic. // Reference. Moscow "Medicine" 1987; 368 p. |
| [11] | Morozov S.A., Petrov M.M., Varlamova C.B. et al. Morphological and functional features of autoerythrocytes in preoperative harvesting in hematological patients // [Journal] Hematology and transfusiology. - 2010. - No. 6.- p. 58. |
| [12] | Panov V.P. The blood component standard "Erythrocyte mass - raw materials for obtaining blood products" / V.G1. Panov, A.V. Karyakin, Yu.S. Nezhichik // Problems of Hematology. -2003. - №1. - S. 52-53. |
| [13] | Ragimov A.A. Present, problems and prospects of transfusiology. / // Vestnik RAMS. - 2012. - No. 10. - S.70-76. |
| [14] | Yusupova L.B. On increasing the accuracy of determining the activity of erythrocyte glutathione reductase activity. Lab affair 1989; 4: 19-21. |
| [15] | Khalaichev E.E. Optimization of erythrocyte cryopreservation programs for the reservation of hemotherapeutic agents // Diss. Cand. honey. Sciences. - SPb., 2009. - 118 p. |
| [16] | Gajic O., Yilmaz M., Iscimen R. et al. Transfusion from male-only versus female donors in critically ill recipients of high plasma volume components // [Journal] Crit. Care Med.. - 2007. - №7: Vol. 35. - pp. 1645-1648. |
| [17] | Holland L.L., Brooks J.P. Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results // [Journal] Am J ClinPathol. - 2006. - 1: Vol. 126. - pp. 133-139. |
| [18] | Leal-Noval S.R., Arellano-Orden V., Maestre-Romero A. et al. Impact of national transfusion indicators on appropriate blood usage in critically ill patients // [Journal] Transfusion. - 2011. - 9: Vol. 51. - pp. 1957-1965. |
| [19] | Shehata N., Burns L.A., Nathan H. et al. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery // [Journal] Transfusion. - 2012. - №1: Vol. 52. - pp. 91-99. |
| [20] | Van de Watering L. M. G. Clinical effects of transfusing older red cell concentrates: an updated overview // [Journal] ISBT Science Series. - 2012. -Vol. 7. - pp. 235-237. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML