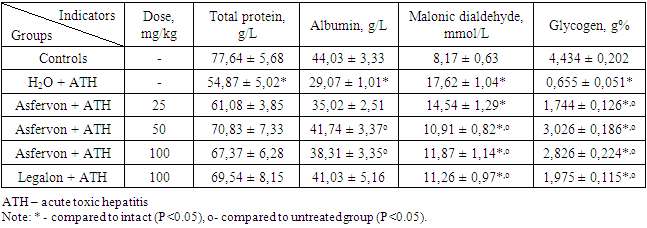-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(9): 728-732
doi:10.5923/j.ajmms.20201009.21
Received: Aug. 20, 2020; Accepted: Sep. 12, 2020; Published: Sep. 15, 2020

Hepatoprotective Activity of Gum Resin of Ferula Assa-Foetida
Z. Z. Khakimov1, A. Kh. Rakhmanov1, Sh. T. Safaeva2
1Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan
2Urgench Branch of the Tashkent Medical Academy, Uzbekistan
Correspondence to: A. Kh. Rakhmanov, Pharmacology and Toxicology Department, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A model of acute toxic hepatitis was reproduced by carbon tetrachloride (50% emulsion in olive oil 0.5 ml/100 g) in order to study the hepatoprotective activity of the resin of Ferula (Ferula assa-foetida L.) on male white rats an initial weight of 165-185 g. The results of the studies showed that, Asfervon (25, 50 and 100 mg/kg) has a pronounced hepatoprotective activity which decrease the activity of liver enzymes in the blood serum. The preventive administration of Asfervon, in comparison with Legalon, prevents the development of intrahepatic cholestasis, impaired metabolism of bilirubin, total protein and albumin in animals with acute toxic hepatitis. It has a expressed antioxidant activity, which is manifested in the suppression of the formation of products of free-radical oxidation of lipids, and it decreases the permeability of the plasmatic membrane of hepatocytes while maintaining the activity of the membrane bound enzymes. The resin of Ferula assa-foetida L. leads to the preservation of glycogen deposits in the liver by its antioxidant properties and a positive effect in the prevention of metabolic disorders, which undoubtedly plays an important role in the provision of synthetic processes with energy resources. It is considered possible to further develop a medication based on this plant as an effective agent in the prevention and treatment of pathology of the hepatopancreatobiliary system.
Keywords: Acute toxic hepatitis, Hepatoprotectors, Gum - resins of Ferul asafoetide, Enzymes
Cite this paper: Z. Z. Khakimov, A. Kh. Rakhmanov, Sh. T. Safaeva, Hepatoprotective Activity of Gum Resin of Ferula Assa-Foetida, American Journal of Medicine and Medical Sciences, Vol. 10 No. 9, 2020, pp. 728-732. doi: 10.5923/j.ajmms.20201009.21.
Article Outline
1. Introduction
- The liver is the central organ in maintaining the body's chemical homeostasis due to its significant participation in various types of metabolism. Therefore, the preservation of the functional activity of the organ allows adequately to a high degree to ensure the vital activity of the internal organs. Along with the synthetic processes in the liver, there are processes of detoxification of exogenous and endogenous toxic substances, perverted metabolism in various pathological conditions. At the same time, the liver is the main store of glycogen being the main organ of energy supply. Any extreme body condition requiring an urgent increase in energy resources supply cannot happen without liver involvement.The health care system is one of the important sectors of the national economy that ensures the safety of the population's health. The latter largely depends on environmental factors, lifestyle, as well as the impact of stressful or extreme conditions of technogenic and natural origin. In terms of preserving the various functions of hepatocytes and in order to ensure chemical homeostasis, the use of hepatoprotective drugs seems to be important. Therefore, in order to prevent liver damage, it is necessary to use not only traditional folk remedies but also physiologically active hepatoprotective substances.The socio-economic development of each country is determined by the quantity and quality of drugs produced in the country itself, its large stock. For in extreme conditions, such as a pandemic, natural disasters, earthquakes, etc., the supply and provision of vital medicines to patients can be significantly disrupted.Hepatoprotective medications designed to prevent lesions of hepatocytes under the influence of factors of infectious and non-infectious origin is one of the most frequently prescribed medications in this field. However, the pharmacotherapeutic activity of the drugs used in medical practice for the prevention and treatment of liver disorders in the Republic is considered insufficient [1]. Although the number of pharmacological agents used in the complex treatment of liver, biliary tract and pancreatic diseases exceeds 1000 titles, however, those substances which have an evidence base of improved liver function are a relatively small group [1,2].Hepatoprotective drugs are complex substances, designed to increase the liver's resistance to toxic effects, helping to restore its functions, normalizing or enhancing the activity of hepatocyte enzymatic activity [3,4]. The most often use of hepatoprotective substances is plant origin [5]. The hepatoprotective effect of plant extracts is provided by the presence in their composition of phenol carboxylic acids, coumarins, flavonoids with are pharmacologically active [6]. In this regard, the study of the hepatoprotective effect of extracts obtained from plant materials is an important task. Moreover, most of them are obtained from abroad. However, the abundance of plant materials in Uzbekistan is a good platform to create an effective and safe hepatoprotective agent. In this regard, we were interested in the study of gum resin Ferula assa-foetida in acute toxic hepatitis. In traditional medicine, this plant was used to treat spasms, convulsions, hysteria, hypochondria, bronchial asthma, pulmonary tuberculosis, eczema, syphilis, malignant neoplasms, externally as a wound healing tool, neurasthenia, cold and flu, treatment of the prostate inflammation, diabetes mellitus, etc.) [7,8].A comprehensive pharmacological study of a newly available, local medication that may restore or improve the function of hepatocytes in pathological conditions of various etiologies, which is created from Uzbekistan plants has a potentially beneficial effect in terms of affordability, safety, and effectiveness.The purpose of the study was to investigate the hepatoprotective activity of Legalon compare to gum resin Ferula assa-foetida in rats with acute toxic hepatitis induced by carbon tetrachloride.
2. Material and Methods
2.1. Plant Material and Preparation of Dried Extract
- The technology of obtaining gum resin from plant Ferula assa-foetida L. was titled “Asfervon”, and the stages of its creation are described below. To obtain “Asfervon”, we used a plant growing in the Tashkent region. Five-year-old plants were selected that did not release the stems. The soil around its root was removed up to 20 cm in diameter and up to 15 cm deep, thereby revealing the root for further work. Further, in order to protect the root from direct sunlight and dust, the root was covered with a prepared “roof” made of cardboard. From the aerial part of the root, from the lateral side towards the center, an incision was made with a length of 2-3 cm and a thickness of 2 to 3 mm. After that, milky sap that hardened in the air was collected from the section of the aerial part of the root, which is called the gum resin "asafoetida". The milky juice that accumulated and solidified in 3-4 days was collected, and then a repeated transverse incision was made. This process was repeated until the end of the appearance of milky sap in the passages of the phloem part of the root, as well as depending on changes in climatic factors (temperature, precipitation, wind). At the end of the collection, the root was completely covered with soil so that the plant did not lose its physiological properties. An average of 900-1300 g of milky juice was obtained from one plant. The upper part of the hardened milky juice was yellowish, and the inside was milky-creamy. The solidified milky juice was crushed on a mechanical stirrer to a powdery state. The resulting powder was treated with 90% ethyl alcohol in a mass ratio of 1:10 for 40 minutes with constant stirring, the mass was filtered from the accompanying substances on a putsch filter under vacuum. The purified milky juice eventually looks like a powder of a milky-cream color with a specific odor, which consists of resin on 60-70%, 22-35% was gum, and essential oil was around 2-5%. The essential oil was composed of organic sulfides, pinene, p-hydroxycoumarins, and other compounds. The gum resin contained the sum of esters of sesquiterpene alcohols, ferulic acid, sesquiterpene alcohols, coumarin, umbelliferone, and trace amounts of vanillin [9,10].
2.2. Experiments
- Sexually mature male rats with an initial weight of 165-185 g were used for the experiment. The animals were kept under established sanitary rules for the design, equipment, and maintenance of experimental biological clinics of Uzbekistan, the rules and regulations of a quality laboratory (GLP) for preclinical studies, as well as International Recommendations of the European Convention for the Protection of Vertebrate Animals used in Experimental Research (1986). The animals were fed with natural and briquettes food, in accordance with the normal guidelines. After purchasing, the animals were quarantined and acclimatized in a vivarium for 14 days. Experimental groups of animals were formed by 6 animals in each, taking into account the body weight. Acute toxic hepatitis (AHT) was reproduced by subcutaneous injection of a 50% emulsion of carbon tetrachloride in olive oil was prepared and injected subcutaneously at the rate of 0.5 ml / 100 g body weight for 4 days. One day before the administration of hepatotoxin and on the following days, within 1 hour of administration of hepatotoxin, the animals were divided into several groups, which were injected intragastrically with a freshly prepared aqueous suspension of Asfervon at doses of 25, 50 and 100 mg/kg intragastrically. A separate group of animals received Legalon at a dose of 100 mg / kg intragastrically. The control group of rats received a similar volume of olive oil emulsion with water intragastric and subcutaneously. Approximately 24 hours after the last injection of drugs and toxin, the animals were decapitated under light anesthesia, blood was collected for biochemical studies, and the liver was removed to determine the glycogen content. Determination of the glycogen content in the liver, as well as the products of lipid peroxidation in the blood, malondialdehyde (MDA), was carried out by the previously described method [11].In the model of toxic liver damage, the hepatoprotective properties of Asfervon were assessed by determining various biochemical markers characterizing liver function such as total protein, albumin, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamine transferase (GGT) and alkaline phosphatase (ALP). Biochemical studies were carried out photometrically on a semiautomatic biochemical analyzer Mindray (China, 2014) using kits from Human (Germany) and Cypress diagnostics (Belgium).All experiments were performed in compliance with the requirements of the European Convention "On Protection of vertebrate animals used for experimental and other scientific purposes" (Strasbourg 1986).
2.3. Statistical Analysis
- The received results were subjected to the statistic processing with the using of standard software package Biostat 2009 on well-known method of variation statistics with an estimation of the statistical significance of indicators (M±m) and differences between groups were analyzed using the Student’s t-test. P<0.05 was considered significant.
3. Results and Discussion
- Hepatoprotective medication is usually a complex substance, mainly of plant origin, designed to increase the liver's resistance to toxic effects, helping to restore its functions, normalizing or enhancing the activity of hepatocyte enzymes [3,4]. Carbon tetrachloride is used in experimental works as a model of acute toxic liver damage [12]. Developing liver failure associated with intensification of peroxidation processes, production and accumulation of highly toxic metabolites, leads to necrosis of hepatocytes and liver fibrosis [13]. According to modern concepts, free radical reactions play a significant role in the development of pathology with toxic liver damage. Reactive oxygen species cause an increase in the intensity of lipid peroxidation of cell membranes and, as a consequence, a violation of its permeability function, which leads to the release of enzyme molecules into the bloodstream. In clinical practice, so-called "liver tests" are usually used such as ALT, AST, ALP and GGT. Proceeding from this, we investigated the activity of enzymes in the serum of rats with acute toxic hepatitis, induced by carbon tetrachloride, preemptively receiving the investigated drugs in a comparative aspect.Thus, in untreated animals, after subcutaneous administration of hepatotoxin, the activity of ALT in the blood serum increases by almost 3 times, and AST by 137.9%, which indicates damage to the plasma membranes of hepatocytes. In contrast, in rats preemptively treated with Asfervon, the enzyme activity was low. From the data in Table 1, it can be seen that the drug at a dose of 25 mg / kg inhibits the activity of ALT by 39.2%, and at doses of 50 and 100 mg / kg by 57.2 and 55.4%, respectively, compared to untreated animals. At the same time, the activity of the AST enzyme was less pronounced and in the indicated doses it was only 13.6 - 22.9%. Against this background, Legalon showed almost the same effect as Asfervon.Consequently, in terms of its effect on the activity of liver enzymes, Asfervon is not inferior to the well-known classical hepatoprotective medication as Legalon. It should be noted that liver damage caused by carbon tetrachloride leads to significant structural changes in the liver parenchyma, which is accompanied by cholestatic syndromes, as indicated by the high activity of alkaline phosphatase [14,15]. At the same time, the Asfervon suppressed the liver enzyme activity by 14.6, 47.0, and 46.2%, respectively, at doses of 25, 50 and 100 mg / kg. There was no statistically significant difference in the alkaline phosphatase activity in rats receiving Asfervon and Legalon. We found a similar pattern determining the GGT activity (see Table 1). There was an increased activity of GGT which is involved in the exchange of amino acids. Despite the fact that the activity of this enzyme is most pronounced in the kidneys, the source of the serum enzymes is mainly because of the hepatobiliary system. Therefore, an increase in serum GGT which is the most sensitive indicator shows a disease function of the hepatobiliary system. GGT is also considered as a marker of cholestasis [15,16].
|
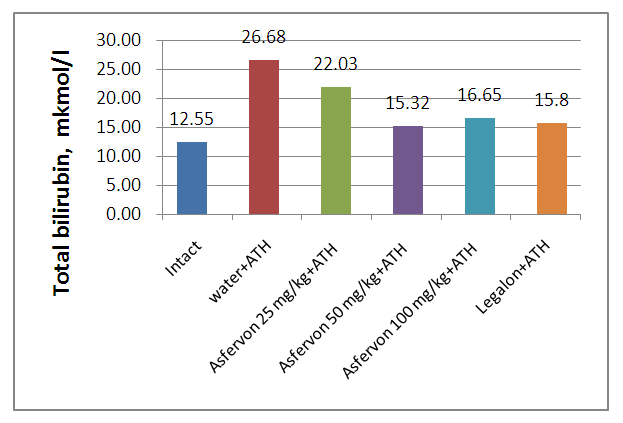 | Figure 1. Preventive Asfervon and Legalon activity on total bilirubin in the serum of rats with acute toxic hepatitis |
4. Conclusions
- 1. Asfervon showed hepatoprotective activity by decreasing liver enzymes in rats with acute liver damage.2. Administration of Asfervon in comparison with Legalon prevents the development of intrahepatic cholestasis, impaired metabolism of bilirubin, a decrease of total protein, and albumin in animals with acute toxic hepatitis.3. Asfervon showed antioxidant activity by decreasing free radical oxidation of lipids by maintaining the activity of the membranes of bound enzymes.4. Asfervon also showed a protective effect on the liver function of glycogen storage.
References
| [1] | Daminov T.A., 2008, Essentiale in the complex treatment of patients with viral hepatitis, Medical Journal of Uzbekistan, 4, p.74-76. |
| [2] | Jadeja R, Devkar R.V., Nammi S. et al., 2014, Herbal medicines for the treatment of nonalcoholic steatohepatitis: current scenario and future prospects, Evid. Based Complement Alterna Med, p.648-658. |
| [3] | Oparin A.G., Lavrova N.V., Blagoveshchenskaya A.V., 2016, Hepatoprotectors: tactics of clinical use, East European Journal of Internal and Family Medicine, 1, p.75-81. |
| [4] | Bibik E.Yu., Shipilova N.V., Krivokolysko B.S. et al., 2019, Features of the pharmacological properties of modern hepatoprotectors, Morphological almanac named after V.G. Koveshnikov, 17(4), p.101-110. |
| [5] | Shabanov P.D., 2010, Effects of the polyprenol drug ropren on toxic damage to the liver and brain in rats: study of the functional state of the liver, behavior and metabolism of monoamines in the brain, Reviews on clinical pharmacology and drug therapy, 8(3), p.7-30. |
| [6] | Eesha B.R. Hepatoprotective activity of Terminalia paniculata against paracetamol induced hepatocellular damage in Wistar albino rats, Asian. Pac. J. Trop. Med. -2011. -Vol.4, No.6, -P.466-469. |
| [7] | Orazova E.A., 2017, Biological characteristics of smelly ferula (COMUC, FERULA FOETIDA) growing on the territory of Turkmenistan, Problems of modern integration processes and ways to solve them. Collection of articles of the International scientific-practical conference. Kazan, part 1, p.7-8. |
| [8] | Amalraj A., Gopi S., 2017, Biological activities and medicinal properties of Asafoetida: A review, Journal of Traditional and Complementary Medicine, 7, p.347-359. |
| [9] | Khakimov Z.Z., Rakhmanov A.Kh. A.A. Tulyaganov, 2018, Preclinical study of the safety of Asfervon, Pharmaceutical Bulletin of Uzbekistan, 1, p. 69-74. |
| [10] | Tulyaganov S.A., Nabiev A., 2017, A method of obtaining an agent with anti-inflammatory and prostatoprotective action, Patent UZ IAP. 5453 Registered in Tashkent on August 24. |
| [11] | Mavlanov Sh.R., Khakimov Z.Z., Rakhmanov A.Kh., 2017, Influence of lesbokhol on the glycogen content in the liver in case of its acute toxic damage, Infection, immunity and pharmacology, 1, p.129-134. |
| [12] | Ivanova V.V., Ligostaeva Yu.V., Poteryaeva O.N. et al., 2013, Study of the hepatoprotective effect of a plant extract of birch bark in experimental hepatitis caused by carbon tetrachloride, Fundamental research, 3, p.277-279. |
| [13] | Kravchenko L.V., 2009, Characterization of the acute toxic effect of carbon tetrachloride as a model of oxidative stress, Toxicological Bulletin, 1, p.12-18. |
| [14] | Khakimov Z.Z., Fayzieva Z.T., Makhmudov S.S., 2017, The effect of celagrip, an interferon inducer on the hepatobiliary system, Tashkent, 130 p. |
| [15] | Biochemical markers of cytolysis syndrome, cholestasis, Cyber Pedia. cyberpedia.su/7x5e9f.html. |
| [16] | Malinin M.L., The reasons for the increase in the activity of gamma glutamine transferase (GGT) in the blood serum. my-volga.ru/content/prichiny -... |
| [17] | Kushnerova N.F., Fedoreev S.A., Fomenko S.E. et al., 2014, Hepatoprotective properties of isoflavonoids from the roots of MAACKIA AMURENSIS in experimental liver damage with carbon tetrachloride, Experimental and Clinical Pharmacology, 77(2), p.26-30. |
| [18] | Zverev Ya. F., 2017, Flavonoids through the eyes of a pharmacologist. Antioxidant and anti-inflammatory activity, Reviews on clinical pharmacology and drug therapy, 15(4), p.13. |
| [19] | Chandran S., Sakthivel M., Thirumavalavan M. et al., 2017, A facile approach to the isolation of proteins in Ferula asafoetida and their enzyme stabilizing, anti-microbial and antioxidant activity, Int. J. Biol. Macromol., 102, p. 1211-1219. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML