-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(9): 664-668
doi:10.5923/j.ajmms.20201009.08
Received: July 3, 2020; Accepted: August 2, 2020; Published: August 26, 2020

Acetylation Phenotype as a Criterion for Predicting the Outcomes of Living Related Liver Transplantation and Immunosuppressive Therapy
Nazirov F. G., Ibadov R. A., Omonov O. A., Ibragimov S. Kh.
Republican Specialized Scientific-Practical Medical Center of Surgery Named after Academician V. Vakhidov, Tashkent, Uzbekistan
Correspondence to: Ibragimov S. Kh., Republican Specialized Scientific-Practical Medical Center of Surgery Named after Academician V. Vakhidov, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective. To evaluate of methods for predicting the post-transplant outcomes and individual selection of the immunosuppression regimen in the early and long-term period in recipients after living related liver transplantation. Material and methods. The prospective study. We analyzed the main clinical results of the early period (6 months) of 9 recipients after related liver transplantation, taking into account the combination of recipient and donor acetylation phenotypes (AcP) - acetylation reactions are catalyzed by N-acetyltransferases, which are localized mainly in hepatocytes. Results. According to our study, fast AcP was detected in 4 recipients, slow - 5. Among 9 pairs of donor-recipient, the following 4 types of relationships between their AcP were revealed: fast AcP of the donor with fast AcP of the recipient (group I) - this combination was observed in most cases – 4. Fast AcP of the donor with slow AcP of the recipient (group II) - in 2 cases. Slow AcP of the donor with fast AcP of the recipient (group III) - 2 observations. Slow AcP of the donor with slow AcP of the recipient (group IV) - was observed in 1 cases. In group, where there was a combination of slow AcP, both from the side of the donor and from the side of the recipient, relatively higher levels of aminotransferases were observed than in other groups throughout the study period. By the time the recipients were discharged, the dynamics of the decrease in ALT and AST was equal, which was associated with the dosage adjustment of immunosuppressive therapy, but it had the lowest values in group I and II. The most pronounced dynamics of changes in the main biochemical parameters of blood reflecting the function of the transplanted liver in the postoperative period was revealed in groups I and II, which were also characterized by a lower frequency of delayed transplant functions, however, the need for increased immunosuppression and prevention of rejection was higher. For selecting the optimal immunosuppressive therapy regimen, a “Method for conducting immunosuppressive therapy after transplantation of solid organs”. Conclusions. The acetylation phenotype, in particular, the relationship between the combinations of donor and recipient acetylator statuses, plays a significant role in predicting the course of the early period after related liver transplantation and immunosuppressive therapy.
Keywords: Living related liver transplantation, Acetylation phenotype, Early posttransplant period, Immunosuppressive therapy
Cite this paper: Nazirov F. G., Ibadov R. A., Omonov O. A., Ibragimov S. Kh., Acetylation Phenotype as a Criterion for Predicting the Outcomes of Living Related Liver Transplantation and Immunosuppressive Therapy, American Journal of Medicine and Medical Sciences, Vol. 10 No. 9, 2020, pp. 664-668. doi: 10.5923/j.ajmms.20201009.08.
1. Introduction
- The introduction of liver transplantation into clinical practice allows to significantly increase the duration and quality of life of patients with liver cirrhosis (LC). The severity of the initial state of the recipient, the duration and invasiveness of the surgical intervention and the function of the donor liver impaired in the early stages, mandatory immunosuppression become objective prerequisites for the development of numerous complications [1-3].It is believed that each recipient in the postoperative period exhibits at least one complication, while their types and etiology are very diverse and most of them are noted in the early time after liver transplantation. Despite adequate immunosuppressive therapy, up to 80% of patients in the interval 7-14 days after surgery have an acute transplant rejection [3,4].The problem of predicting the clinical course of the post-transplant period has been the subject of a few studies; information on the features of postoperative monitoring of such patients is sporadic and not systematized. There is no data on the relationship between the development of complications in patients undergoing liver transplant and the prognostic significance of laboratory parameters reflecting liver and kidney function. The relationships between the parameters characterizing the preoperative status of the recipient, the features of the functioning of the graft with the clinical picture in the postoperative period are not described [4]. Currently, there is a great scientific and practical interest in genetically determined risk factors, which allows us to outline ways to improve the effectiveness of transplantation and creates the basis for predicting the likely outcome of a liver transplant in relation to each recipient. In particular, by identifying a genetic polymorphism of N-acetyltransferase isoenzymes, groups with a normal metabolism, a slow metabolism, and a super-intense metabolism are isolated. Accordingly, there is a danger of the ineffectiveness of immunosuppressive therapy with super-intensive metabolism and, conversely, the occurrence of toxic manifestations with a slow metabolism [5,6,7].The main objectives of this study were the development and evaluation of methods for predicting the post-transplant outcomes and individual selection of the immunosuppression regimen in the early and long-term period in recipients after living related liver transplantation while ensuring accessibility and ease of implementation.
2. Material and Methods
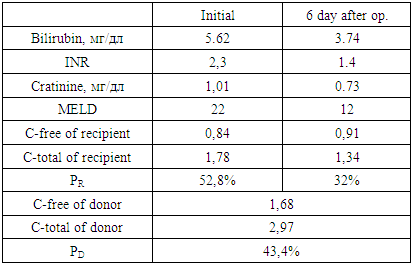
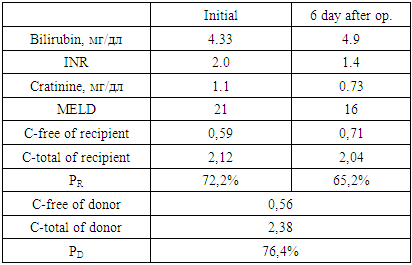
- In this aspect, we proposed a “Method for predicting the course of the early postoperative period after related liver transplantation”, which includes examining the urine of the recipient and the donor before surgery, determining the activity of N-acetyltransferase, assessing the severity of the recipient’s condition on the MELD scale, then substituting the corresponding score for each from the values of the following special table and the results obtained, calculate the risk of complications by the formula:
 where: MELD - the severity of the recipient's condition on the MELD scale before surgery;PD — donor acetylator status (% AC);PR — recipient acetylator status (% AC);if RISK is 3-5 - they predict low, 6-7 points - medium and 8-9 points - a high risk of complications in the early postoperative period.
where: MELD - the severity of the recipient's condition on the MELD scale before surgery;PD — donor acetylator status (% AC);PR — recipient acetylator status (% AC);if RISK is 3-5 - they predict low, 6-7 points - medium and 8-9 points - a high risk of complications in the early postoperative period.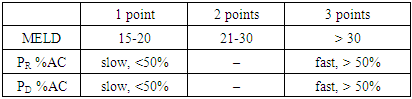 The use of the MELD scale allows one to judge the degree of progression of liver pathology and assess the severity of the condition of patients with liver diseases on the basis of the logarithmic dependence of indicators of the international normalized ratio (INR), bilirubin and creatinine in blood serum.The method is as follows. Acetylator status (the true intensity of detoxification in the liver) is determined by assessing the degree of activity of the N-acetylation reaction by conducting stress tests. For this, the recipient and the donor during hospitalization, in parallel with the standard examination, are invited to take the drug Sulfanilamide at a rate of 50 mg / kg, after 7 hours urine is collected and the concentrations of total (C-total) and free sulfanilamide (C-free) are determined. Further calculations of %AC are carried out according to the formula: %AC=(C-free - C-total)*100%/C-totalAt %AC > 50% - the patient is considered to be a fast acetylator, at %AC < 50% - slow.Assessment of the severity of the condition of patients with liver diseases is carried out according to the MELD scale. To do this, the indicators of INR, bilirubin and creatinine in the blood serum are inserted into the formula:MELD = 3.78 × ln [bilirubin (mg/dl)] + 11.2 × ln [INR] + 9.57 × ln [creatinine (mg/dl)] + 6.43Then, according to the table, a score is determined for each of the obtained indicators, and according to the results obtained, the risk of developing complications is calculated according to the above formula.We analyzed the main clinical results of the early period (6 months) of 9 recipients after related liver transplantation, taking into account the combination of recipient and donor acetylation phenotypes (AcP) - acetylation reactions are catalyzed by N-acetyltransferases, which are localized mainly in hepatocytes.According to our study, fast AcP was detected in 4 recipients, slow - 5. Among 9 pairs of donor-recipient, the following 4 types of relationships between their AcP were revealed:- fast AcP of the donor with fast AcP of the recipient (group I) - this combination was observed in most cases - 4;- fast AcP of the donor with slow AcP of the recipient (group II) - in 2 cases;- slow AcP of the donor with fast AcP of the recipient (group III) - 2 observations;- slow AcP of the donor with slow AcP of the recipient (group IV) - was observed in 1 cases.In order to assess the function of the liver transplant in recipients depending on the combination of AcP immediately after the operation and in the near term, we studied the dynamics of ALT and AST as the main indicators of liver function and the effectiveness of the standard three-component (tacrolimus, sodium mycophenolate and corticosteroids) immunosuppressive therapy.
The use of the MELD scale allows one to judge the degree of progression of liver pathology and assess the severity of the condition of patients with liver diseases on the basis of the logarithmic dependence of indicators of the international normalized ratio (INR), bilirubin and creatinine in blood serum.The method is as follows. Acetylator status (the true intensity of detoxification in the liver) is determined by assessing the degree of activity of the N-acetylation reaction by conducting stress tests. For this, the recipient and the donor during hospitalization, in parallel with the standard examination, are invited to take the drug Sulfanilamide at a rate of 50 mg / kg, after 7 hours urine is collected and the concentrations of total (C-total) and free sulfanilamide (C-free) are determined. Further calculations of %AC are carried out according to the formula: %AC=(C-free - C-total)*100%/C-totalAt %AC > 50% - the patient is considered to be a fast acetylator, at %AC < 50% - slow.Assessment of the severity of the condition of patients with liver diseases is carried out according to the MELD scale. To do this, the indicators of INR, bilirubin and creatinine in the blood serum are inserted into the formula:MELD = 3.78 × ln [bilirubin (mg/dl)] + 11.2 × ln [INR] + 9.57 × ln [creatinine (mg/dl)] + 6.43Then, according to the table, a score is determined for each of the obtained indicators, and according to the results obtained, the risk of developing complications is calculated according to the above formula.We analyzed the main clinical results of the early period (6 months) of 9 recipients after related liver transplantation, taking into account the combination of recipient and donor acetylation phenotypes (AcP) - acetylation reactions are catalyzed by N-acetyltransferases, which are localized mainly in hepatocytes.According to our study, fast AcP was detected in 4 recipients, slow - 5. Among 9 pairs of donor-recipient, the following 4 types of relationships between their AcP were revealed:- fast AcP of the donor with fast AcP of the recipient (group I) - this combination was observed in most cases - 4;- fast AcP of the donor with slow AcP of the recipient (group II) - in 2 cases;- slow AcP of the donor with fast AcP of the recipient (group III) - 2 observations;- slow AcP of the donor with slow AcP of the recipient (group IV) - was observed in 1 cases.In order to assess the function of the liver transplant in recipients depending on the combination of AcP immediately after the operation and in the near term, we studied the dynamics of ALT and AST as the main indicators of liver function and the effectiveness of the standard three-component (tacrolimus, sodium mycophenolate and corticosteroids) immunosuppressive therapy.3. Results
- In recipients with fast AcP from a donor with fast AcP (group I), a significant and rapid decrease in the levels of AST on the 3rd day - 791.0±14.2 IU/L, ALT – 1060.2±54.1 IU/L, while in group II the indices were 745.3±29.5 IU/L and 1147.0±44.2 IU/L, in group III – 810.4±16.6 IU/L and 1162.8±30.2 IU/L, in group IV - 770.2±15.4 IU/L and 1248.8±14.2 IU/L. On the 7th day after the operation, the levels of AST and ALT in group I were 316.2±6.4 IU/L and 591.0±14.5, respectively, and were also significantly lower than in the other groups (p<0.05) (fig. 1).
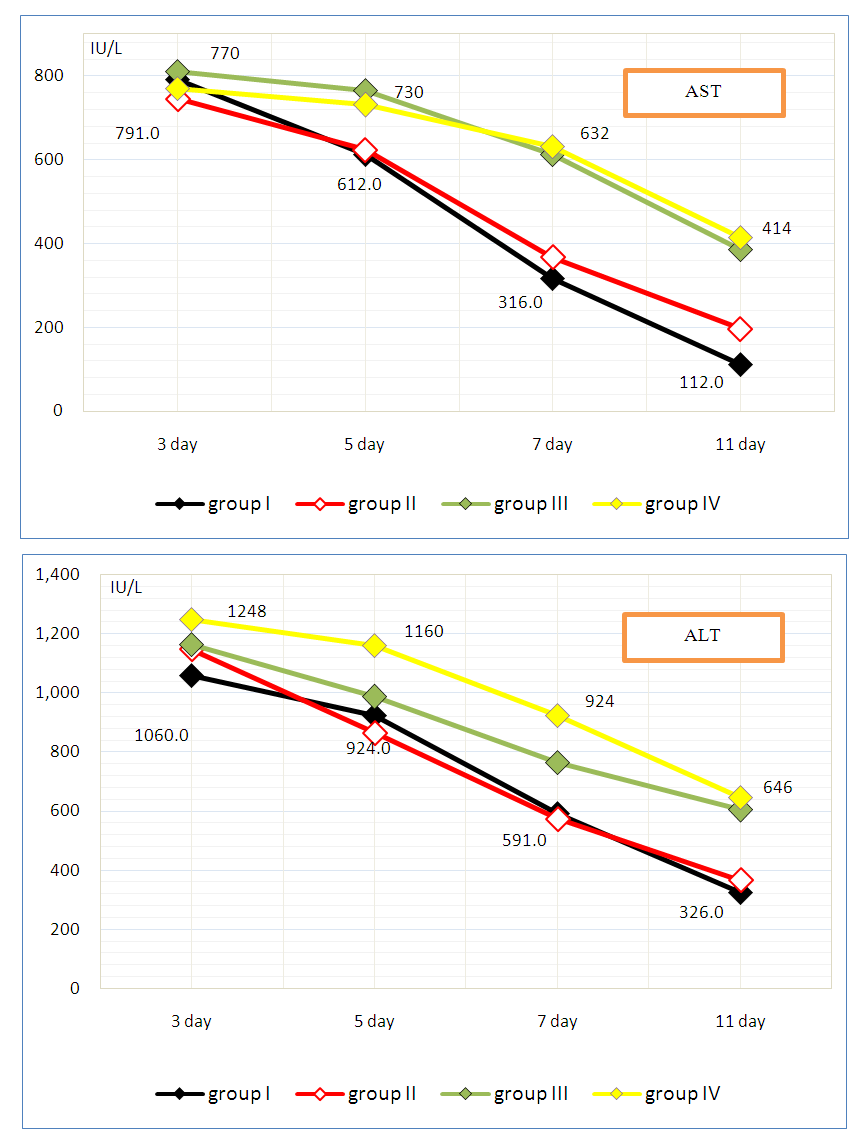 | Figure 1. Dynamics of AST and ALT (IU/L) after related liver transplantation depending on the acetylation phenotype |
|
|
4. Conclusions
- The acetylation phenotype, in particular, the relationship between the combinations of donor and recipient acetylator statuses, plays a significant role in predicting the course of the immediate period after related liver transplantation and immunosuppressive therapy. The principal advantage of fast AcP is noted with respect to the positive dynamics of the main biochemical parameters characterizing the function of the transplanted liver (P <0.05). At the same time, it was determined that fast AcP can serve as a prognostic marker of a predisposition to more rapid development of transplant rejection, which required enhanced immunosuppressive therapy. These circumstances most likely depended on the donor acetylation status.The post-transplantation period of recipients with slow AcP was characterized by a slow restoration of the normal values of hepatic aminotransferases and a greater likelihood of developing delayed graft function than recipients with fast AcP (P <0.05), which in most cases required standard immunosuppressive therapy, and, at the same time, not suggested the development of specific complications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML