-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(8): 627-630
doi:10.5923/j.ajmms.20201008.19
Received: July 16, 2020; Accepted: August 8, 2020; Published: August 15, 2020

Clinical and Microbiological Features of the Use of Black Cumin Oil in the Treatment of Chronic Generalized Periodontitis of Moderate Severity
Rizaev J. A. 1, Gaybullaev E. A. 2, Aloviddinov Sh. D. 3, Jilonova Z. A. 3
1Full Professor, Samarkand State Medical Institute, Samarkand, Uzbekistan
2Associate Professor, Tashkent State Dental Institute, Tashkent, Uzbekistan
3Assistant, Tashkent State Dental Institute, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article substantiates the need for the use of Alkhaday in the treatment of chronic periodontitis of varying severity based on the microbiological landscape of the wound. The aim of the study: To conduct a comparative clinical and microbiological analysis of the landscape of the wound surface of the periodontium after surgical procedures with the use of black cumin oil (Alkhadaya). Materials and research methods: To solve this problem, the results of 120 patients with chronic periodontitis of varying severity were analyzed. Bacteriological research, at the Department of Microbiology of TSDI, subjected to 110 samples of pus, taken from 120 patients before and after treatment, who were admitted to the inpatient department of the Department of Surgical Dentistry of TGSI, with varying severity of periodontitis. Results of the study: According to the results of bacteriological studies of the 120 patients we studied in 23 patients, no anaerobic flora was found in the test material. Anaerobic microorganisms were found in 97 patients (88.3%) in a pure culture and in association both with other anaerobes and optionally with anaerobes and aerobes. Among anaerobes, the overwhelming majority of the selected cultures belonged to bacteroids and fusobacteria; clostridia, peptostreptococcus, and waillonella were much less common. Of the bacteroids, 21 strains were identified as B.oralis, 10-B.asaccharoliticus, 13- B.fragilis, 12- B.melaninogenicus, 10- B.ureoliticus, 14- did not fit into the known identification schemes.
Keywords: Staphylococcus aureus, Porphiromonas gingivalis, Chronic generalized periodontitis, Immunomodulator Alkhaday, Microbiological landscape, Blood agar
Cite this paper: Rizaev J. A. , Gaybullaev E. A. , Aloviddinov Sh. D. , Jilonova Z. A. , Clinical and Microbiological Features of the Use of Black Cumin Oil in the Treatment of Chronic Generalized Periodontitis of Moderate Severity, American Journal of Medicine and Medical Sciences, Vol. 10 No. 8, 2020, pp. 627-630. doi: 10.5923/j.ajmms.20201008.19.
Article Outline
1. Relevance
- As you know, periodontitis is an inflammatory and destructive process of bacterial origin, affecting periodontal tissues and provoking the destruction of the supporting tissues of the tooth. This destructive inflammatory process is essentially the result of an inadequate interaction between the microflora of the oral cavity and the defense mechanisms of the host organism.The leading etiological factor in inflammatory periodontal diseases is currently recognized as microbial plaque (plaque), which contains microorganisms with high pathogenicity, the ability to adhere to tooth tissues and the release of invasive enzymes (endotoxins). WHO defines such a group of microorganisms as periodontopathogenic flora (Porphiromonas gingivalis, Prevotella melanogenica, Veillonella parvula, Fusobacterium nucleatum, Peptostreptococcus micros, etc.) [1-5,11-20].Periodontal bacteria produce enzymes that stimulate the activity of various immunocompetent cells - macrophages, white blood cells. Proteolytic enzymes of periodontal bacteria are one of the most important virulent factors: Actinobacillus actinomycetecomitans and Porphyromonas gingivalis, which have a wide range of virulence factors, in particular proteolytic and osteoresorbing activities. Actinobacillus actinomycetecomitans produce collagenase (collagenolytic activity); Porphyromonas gingivalis - metalloproteinases, cysteine proteinases, asparagin proteinases, causing degradation of non-specific Ig A and Ig G (by splitting them into small peptides) [6-10,21,22].The therapeutic strategy for the treatment of chronic generalized periodontitis should include not only local drugs, but also systemic ones, since the ultimate goal of periodontitis treatment is to keep the teeth functional and comfortable and ensure health at the stages of surgery. One of the supporting immunity at the proper level is black cumin oil (Alkhadaya). Black cumin oil (Alkhadaya) includes a unique complex of biologically active substances: minerals, nigelon, sulfur, phosphorus, iron, calcium, active enzymes, carbohydrates, proteins, vitamins A and E, linoleic acid, oleic, palmitic, eicosen, stearic, alpha-linolenic and fragrant substances - carbon, essential oils.However, in the available literature, the use of black caraway seed oil (Alkhadaya) did not occur in periodontal disease, so it was relevant to conduct a similar study.
2. The Aim of the Study
- To conduct a comparative clinical and microbiological analysis of the landscape of the wound surface of the periodontium after surgical procedures with the use of black cumin oil (Alkhadaya).
3. Materials and Research Methods
- To solve this problem, the results of 120 patients with chronic periodontitis of varying severity were analyzed. Bacteriological research, at the Department of Microbiology of TDSI, subjected to 110 samples of pus, taken from 120 patients before and after treatment, who were admitted to the inpatient department of the Department of Surgical Dentistry of TDSI, with varying severity of periodontitis. The studied material was taken from the pathological site with a sterile syringe and delivered in a special transport medium (TNS), in a ratio of 1:10, which, after preliminary microscopy in order to determine the degree of contamination with the presumed pathogenic substance, was seeded on the following electively selective nutrient media: blood agar (CA) ) - to highlight streptococci and their identification to a species; milk-yolk-salt agar (MZHSA) - for the isolation of staphylococci and their subsequent identification to group and species; Endo medium - to isolate representatives of the Enterobacterium family and their identification to the species; Kita-Tarozzi - to determine the presence of anaerobes with their subsequent identification to the clan or group; Saburo medium - with and without antibiotics - to determine the presence of fungi, and in particular fungi of the genus Candida.The research results were statistically processed using the Student-Fisher test, the non-parametric Mann-Winnie test, the Kraskes-Wallis test, and the Wilconson test.
4. Research Results
- According to the results of bacteriological studies, out of 120 patients we studied, 23 did not find anaerobic flora in the studied material in 23 patients. Anaerobic microorganisms were found in 97 patients (88.3%) in a pure culture and in association both with other anaerobes and optionally with anaerobes and aerobes. Among anaerobes, the overwhelming majority of the selected cultures belonged to bacteroids and fusobacteria; clostridia, peptostreptococcus, and waillonella were much less common. Of the bacteroids, 21 strains were identified as B.oralis, 10-B.asaccharoliticus, 13- B.fragilis, 12- B.melaninogenicus, 10- B.ureoliticus, 14- did not fit into the known identification schemes. Among fusobacteria, 21-F.necroforum, 14-F.nucleatum, 8-F.mortiferum, and 5- were not identified. The most common associates of anaerobes were epidermal, Staphylococcus aureus and hemolytic streptococci. As a result of microbiological research, 34 strains of representatives of the genus Staphylococcus (43.68%) were found; 17 strains of Staphylococcus aureus (48%); 14 strains of Staphylococcus epidermitis (41.2%); 1 Staphylococcuss capitis (3%), 2- Staphylococcus spp (6%). Streptococci were isolated in only 12 cases and represented by Streptococcus pyogenes (10%), 3 - α-hemoliticus streptococcus (30%), 1-β hemolyticus streptococcus (10%), 1-Staphylococcus intermedis, 1-Streptococcus veridans (10%) and 2 Streptococcus spp (20%) (Fig. 1).
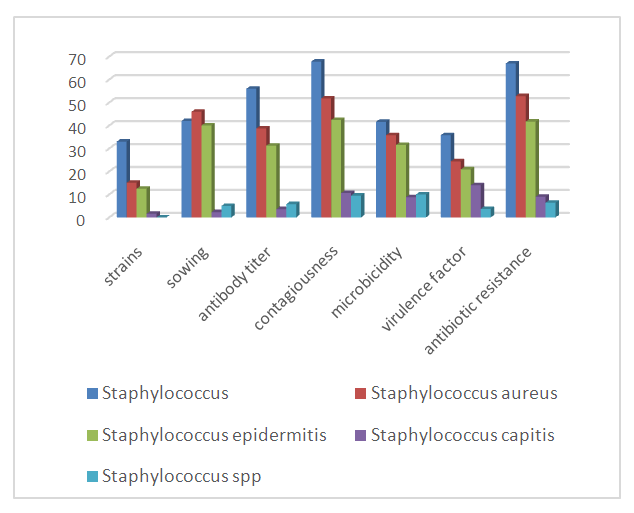 | Figure 1. Data from microbiological studies (staphylococci) in the main and control groups (before treatment) |
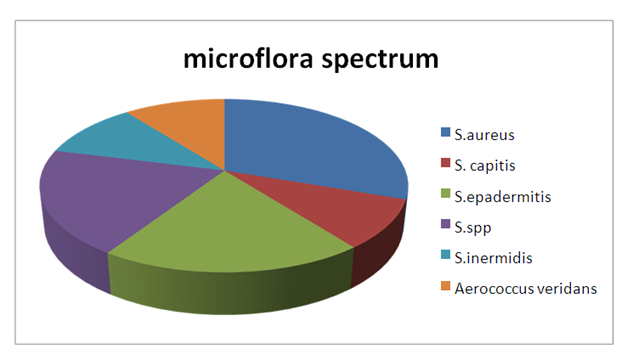 | Figure 2. The spectrum of microflora in patients with chronic generalized periodontitis |
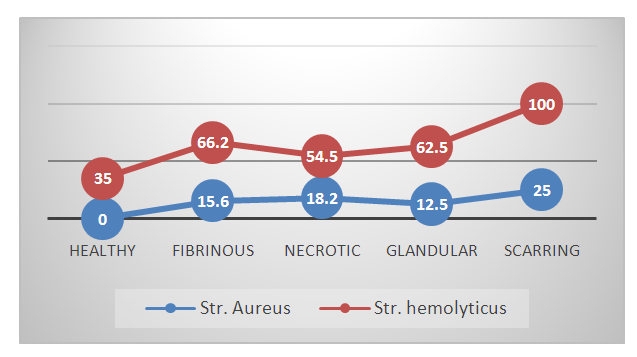 | Figure 3. The prevalence of pathogenic microflora in periodontal patients |
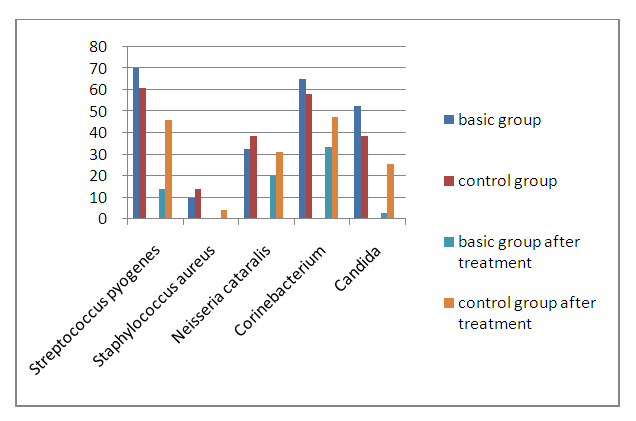 | Figure 4. The dynamics of the oral microbiocenosis of patients with periodontal diseases (chronic generalized periodontitis of moderate severity) before and after treatment |
5. Discussion
- Chronic periodontitis is a common disease of the maxillofacial region. Its aggressive course, pathogen contagiousness, antibiotic resistance, and widespread use at a young age determine the urgency of this problem. Since many periodontopathogenic strains are insensitive to the antibiotics used due to the high virulence of the pathogen and the variable expressivity of these pathogens, it is recommended to include Alkhaday's immunomodulator in antibacterial therapy, since it contains many biologically active elements to increase the nonspecific and specific resistance of the host organism.The study included the results of 120 patients with varying degrees of severity of chronic periodontitis, who were treated at the Department of Surgical Dentistry, TDSI (Tashkent Dental State Institute).Among anaerobes, the overwhelming majority of the selected cultures belonged to bacteroids and fusobacteria; clostridia, peptostreptococcus, and waillonella were much less common. Of the bacteroids, 21 strains were identified as B.oralis, 10-B.asaccharoliticus, 13- B.fragilis, 12- B.melaninogenicus, 10- B.ureoliticus, 14- did not fit into the known identification schemes. Among fusobacteria, 21-F.necroforum, 14-F.nucleatum, 8-F.mortiferum, and 5- were not identified. The most common associates of anaerobes were epidermal, Staphylococcus aureus and hemolytic streptococci. As a result of microbiological research, 34 strains of representatives of the genus Staphylococcus (43.68%) were found; 17 strains of Staphylococcus aureus (48%); 14 strains of Staphylococcus epidermitis (41.2%); 1 Staphylococcuss capitis (3%), 2- Staphylococcus spp (6%). Streptococci were isolated in only 12 cases and represented by Streptococcus pyogenes (10%), 3 - α-hemoliticus streptococcus (30%), 1-β hemolyticus streptococcus (10%), 1-Staphylococcus intermedis, 1-Streptococcus veridans (10%) and 2-Streptococcus spp (20%).After treatment, in the majority of patients who underwent the recommended course of treatment, we found a decrease in both strains, seeding and microbicides of periodontopathogenic bacteria, as well as an improvement in the antibiotic resistance index in treated patients. In general, judging by the dynamics of the recovery process, wound healing was better in patients who were offered Alkhadaya as additional therapy.
6. Findings
- 1. The clinical picture of anaerobic non-clostridial infection of periodontal tissues is distinguished by the severe and / or extremely severe course of the disease (66.9% of patients) with a predominant lesion of all periodontal layers. In addition to the characteristic clinical picture, it is inherent to isolate anaerobic-aerobic microbial associations (73.8%) from the primary infection site, specific morphological changes in periodontal tissues, and a high level of toxic metabolites in the tissues of the infection site.2. After antibiotic therapy with the additional use of Alkhaday’s immunomodulator, the microbiological status improved, which is confirmed by the improvement of the clinical picture in the form of wound healing.
ACKNOWLEDGEMENTS
- I want to express my gratitude to my supervisor, Doctor of Medicine, Prof. Rizayev Zhasur Alimjanovich for a sensitive and subtle understanding of the underlying mechanisms that occur in the maxillofacial region and for the warm attitude to the researcher.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML