-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(8): 606-613
doi:10.5923/j.ajmms.20201008.15
Received: June 21, 2020; Accepted: July 27, 2020; Published: August 15, 2020

Optical Coherence Tomography with Angiography in the Early Diagnosis of Changes in the Organ of Vision in Atherosclerosis
D. K. Makhkamova
(TIPME) Tаshkent Institute of Postgrаduаte Medicаl Educаtion of the Ministry of Health of Republic of Uzbekistan, Parkent, Uzbekistаn, Tаshkent
Correspondence to: D. K. Makhkamova , (TIPME) Tаshkent Institute of Postgrаduаte Medicаl Educаtion of the Ministry of Health of Republic of Uzbekistan, Parkent, Uzbekistаn, Tаshkent.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background. The study of the parameters of optical coherence tomography with angiography (OCTA) broadens the understanding of the state of hemodynamics for the early detection of vascular disorders of the organ of vision in atherosclerosis (AS). Purpose. To study the parameters of optical coherence tomography with angiography in cases of changes in the organ of vision at atherosclerosis. Material and methods. 26 patients (41 eyes) with changes in the organ of vision in AS. The age contingent of patients ranged from 32 to 78 years, the average age was 47.5 ± 2.0 g, of which 10 were women, 16 men. Depending on the damage of target organs, all patients were conditionally divided into 2 groups: group I (19 eyes) included patients without damage, and group II (22 eyes) consisted of patients with target organs damage. Results. The registration of OCTA in patients of group I, a decrease in the density of capillaries of the superficial vascular plexus was 15% and amounted to 45.21 ± 2.62% (p <0.05), of the deep vascular plexus by 19%, which amounted to 45.89 ± 2, 71% (p <0.05). In the macular region, the area of the hypoperfused retina was 1.07 ± 0.14 mm2 (p <0.05). In the region of the optic nerve disc, areas of hypoperfusion were noted in the superficial layers in 7 eyes and in the deep layers in 4 eyes. Analysis of OCTA in patients of group II revealed a sharp decrease (by 48%) in the density of capillaries in both the superficial and deep vascular plexuses of the retina, which amounted to 33.91 ± 3.01% (p <0.05); 33.65 ± 2.89% (p <0.05), respectively. In the macular region, the area of the non-perfused retina was 2.19 ± 0.21 mm2 (p <0.05). In the area of the optic nerve disc, areas of nonperfusion were noted both in the surface layers and in the deep layers in 4 eyes. Conclusion. The use of OCTA allows to detect changes in hemoperfusion in all layers of the retina and optic nerve in the early stages of AS development, which will allow early diagnosis and monitoring of the disease.
Keywords: Optical coherence tomography with angiography, Ischemic diseases, Organ of vision, Atherosclerosis, Hemoperfusion, Vascular plexuses, Retina and optic nerve
Cite this paper: D. K. Makhkamova , Optical Coherence Tomography with Angiography in the Early Diagnosis of Changes in the Organ of Vision in Atherosclerosis, American Journal of Medicine and Medical Sciences, Vol. 10 No. 8, 2020, pp. 606-613. doi: 10.5923/j.ajmms.20201008.15.
Article Outline
1. Introduction
- Destructive changes in the vascular wall underlie the formation of atherosclerotic plaques, aneurysms, parietal and obliterating blood clots, and internal hemorrhages. The occurrence of vascular spasm, thrombosis, embolism can also lead to acute impairment of circulatory system in the brain, heart, and in all structures of the organ of vision [1,3,4,5].Оphthalmic manifestations may be the only predictors of the development of acute circulatory disorders of the brain and heart. Therefore, early detection of disturbances in the hemocirculation of acute respiratory infections is an important task that will prevent the development of formidable diseases that threaten the patient's life [4,5,6].In ophthalmology, methods for evaluating the hemodynamics of the eyeball have been widely used in recent years. one of the highly informative, easily accessible and non-invasive methods is optical coherence tomography with angiography mode (OCT-angiography (OCTA)). This method is characterized by calculating changes in the amplitude of optical rays reflected from tissues. The method is based on measuring the degree of amplitude decorrelation at a certain point in the optical B-scan when performing several consecutive b-scans (split-spectrum amplitude-decorrelation angiography-SSADA), which is the result of changes in the characteristics of scattering and absorption of the beam at a particular scanning point over time.OCTA makes it possible to evaluate perfusion of the optic nerve disc (OND), retina, and choroid. In case of vascular disorders of the organ of vision, the density of peripapillary arteries, veins and capillaries can be weakened both in the superficial vascular plexuses of the disk and in the deeper ethmoid plate. Retinal hemodynamics is evaluated by the concept of «Flow Index» (blood flow index). This index contains information on the density of blood vessels and blood flow velocity (the average value of decorrelation in the studied area of the retina) [7-10]. The prospect of using OCTA is determined by sufficient information content and the safety of the method in the dynamics of the process.The study of OCTA parameters expands the understanding of the state of the vascular plexuses of the retina, optic nerve and choroid, which contributes to a detailed study of hemodynamics for early detection of vascular disorders of organ of vision at AS without the introduction of dye [1-3].
2. Purpose of Research
- To study the parameters of optical coherence tomography with angiography in cases of changes in the organ of vision at atherosclerosis.
3. Material and Methods
- A total of 26 patients (41 eyes) with changes in the organ of vision at AS were examined. The age group of patients ranged from 32 to 78 years, while the average age was 47.5±2.0 y (р<0,05), of which 10 were women and 16 were men. The criterion for inclusion of patients in this study, according to the recommendations of WHO experts, was: the presence of dyslipidemia (increased levels of total cholesterol, triglycerides, lipoproteins), increased body mass index, smoking index, the ratio of the waist and hip circumferences, as well as AS (echographically, angiographically or coronarographically proven) damage to the vessels of the target organs. The exclusion criteria from this study were: the presence of diabetes mellitus, diseases of the organ of vision, acute disturbance of cerebral circulation, malignant and autoimmune diseases.The diagnosis, as well as damage to target organs in atherosclerosis, was established by cardiologists, neurologists and angiosurgeons.For scientific research, the approval of the Ethical Commission for Medical Research of the Ethical and Pharmaceutical Committee of the Republic of Uzbekistan was received in accordance with the Helsinki Declaration of the World Medical Association. Also, for inclusion in the research work, voluntary informed consent of the patients was obtained.Depending on the damage to the target organs (brain and heart), all patients were conditionally divided into 2 groups: the first group (19 eyes) included patients without target organs damage, and the second group (22 eyes) consisted of patients with target organs damage.All patients underwent a comprehensive examination, including a study of central visual acuity, computed static perimetry, tonometry, gonioscopy, biomicroscopy, fundus ophthalmoscopy, ultrasound dopplerography of the vessels of the eye and brachiocephalic trunk. For multimodal assessment of the (morphological and hemodynamic) parameters of optic nerve head and the retina, all patients were examined on optical coherence tomography with angiography of the optic nerve head region (ONH protocol) and the macular region (RNFL, GCC) on the TOPCON Triton plus function (Ver. 10.13) (Swept Source OCT). Scanning was carried out at a speed of 100,000 scans per second. This mode allows to receive OCTA-grams in the following sizes: 3 × 3, 4.5 × 4.5, 6 × 6 and 7.5 × 7.5 mm with 304 × 304 pixels. The retina scans were automatically segmented into “superficial”, “deep” layers of the inner retina; outer retina and choriocapillary layer. A statistical analysis of the data was carried out using the software package Statistica 6.0. The mean value (M), standard deviation (s) were determined. To compare the variational series, the Mann – Whitney, Wilcoxon, and Z-criteria were used. The critical significance level was p <0.05.
4. Results and Discussion
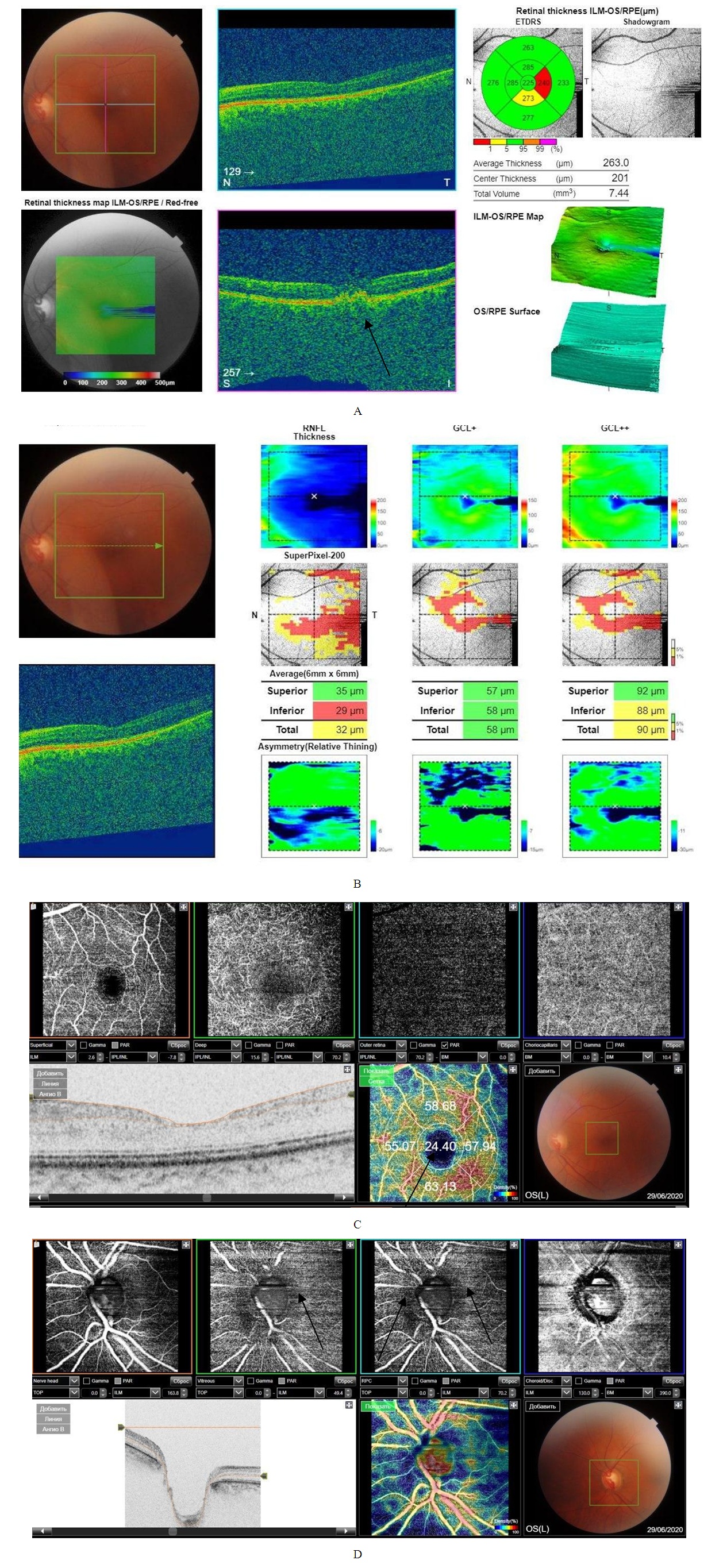
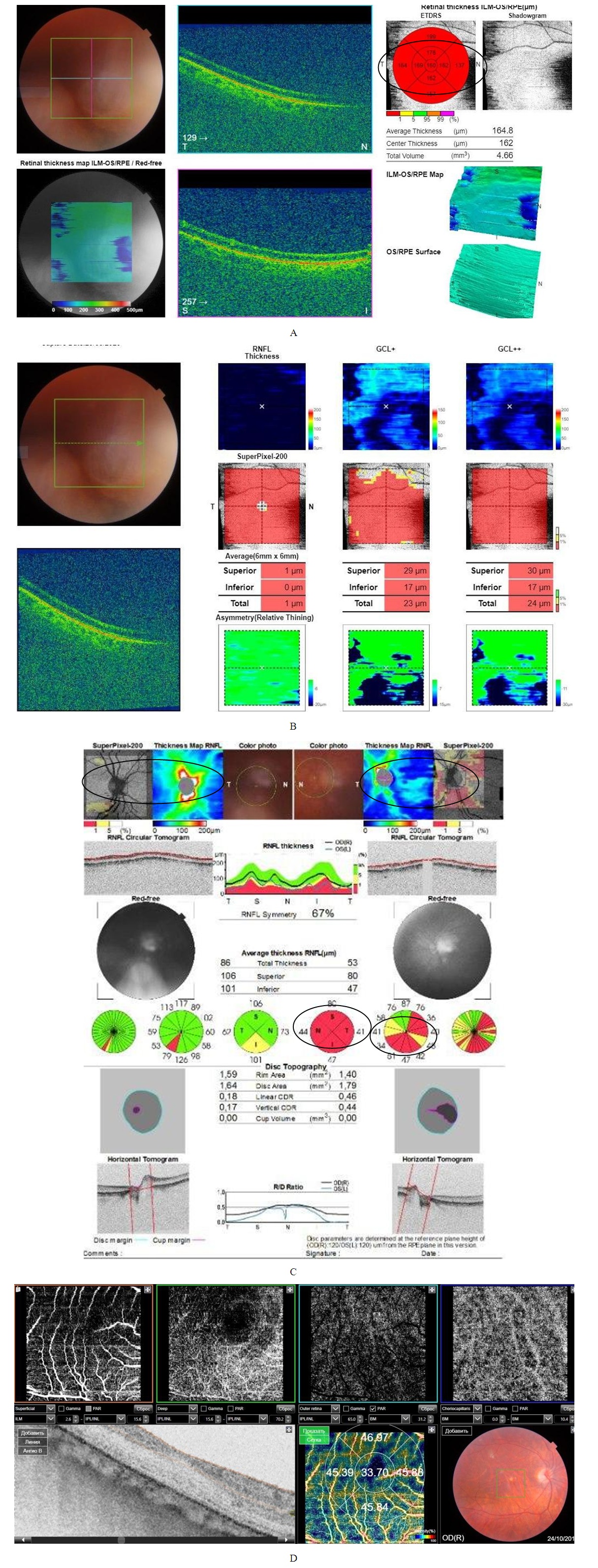
- In the study of visual acuity, the indicators were within normal values and amounted to a maximum correction of 0.87 ± 0.18 (p <0.05) with an average correction, intraocular pressure within 17.41 ± 0.21 mm Hg (p <0.05). When examining the visual fields were determined: in 6 eyes relative scotomas, in 5 eyes a concentric narrowing, in 8 eyes without pathological changes in the visual fields.When recording computer perimetric data a decrease in retinal photosensitivity was noted - MD -2.51 ± 0.11 dB, PSD 3.59 ± 0.09 dB (p <0.05), relative scotomas were observed, an increase in the blind spot area while maintaining high acuity view.During biomicroscopy of the anterior segment of the eyeball, ischemic angiopathy of the first degree limb was determined in 6 eyes, II degree in 5 eyes. Also, in 12 eyes, pupillary reactions were weak.A study of tear production of patients 1 group revealed a decrease in Schirmer's test results, which was within 7.84 ± 0.23 mm (p <0.05). When studying the cytological composition of the lacrimal fluid in patients of the first group, a small monocytosis of 4.01 ± 0.23 was detected (p <0.05). at normal values of acid-base balance, pH 7.61 ± 0.21. In addition, in the study of the S100 ischemia marker [4,5], showed an increase in its number 8 times, which amounted 0.87 ± 0.012 μg /l (p <0.05) with a norm of 0.109 μg /l.Registration of electrophysiological parameters (EPP) of the optic nerve and retina did not reveal pathological changes in latency and amplitude of visual evoked potentials (VEP) and electroretinography (ERG) in patients of group 1.A study on optical coherence tomography revealed a thickening of the layer of retinal nerve fibers (RNFL) of the upper and lower segments in 11 eyes, a thinning of the neuroretinal girdle (NRG) in 7 eyes, and a thinning of the para- and perifocal region in 8 eyes.When registering OCTA in patients of the first group, there was a decrease in the density of capillaries of the superficial layer - the superficial vascular plexuses by 15% and amounted to 45.21 ± 2.62% (p <0.05), of the deep layer - of the deep vascular plexuses by 19%, which 45.89 ± 2.71% (p <0.05). In the macular region, the area of the hypoperfused retina was 1.07 ± 0.14 mm2 (p <0.05). In area optic nerve head, hypoperfusion sites were noted in the superficial layers on 7 eyes, in the deep layers on 4 eyes (Fig. 1).
5. Conclusions
- This study revealed hemoperfusion disorders in all layers of the retina and optic nerve head in the early stages of the development of the AS process, when the target organs were not yet involved. This condition demonstrates the possibility of using the OCTA method for early identification of atherosclerosis in the early stages of the disease, which will prevent the disability of the population and prevent deaths.With the generalization of atherosclerosis and damage to target organs, the use of OCTA allows monitoring of the ischemic process, which contributes to a more adequate management of patients with hemodynamic impairment of organ of vision in AS.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML