K. E. Makhkamov, A. B. Salaev, M. K. Makhkamov, D. U. Israilov
Republican Research Centre of Emergency Medicine
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Introduction The problem of treating patients with severe cranio-cerebral injury remains relevant in modern medicine and has great socio-economic importance. The main contingent of patients is people of the most working age up to 40-50 years. The increase in disability and the costs of subsequent rehabilitation bring economic damage to any country in the world which determines the need to develop new methods of treatment and diagnostics. Aim of the study is a comparison of the surgical treatment results of patients with severe cranio-cerebral injury and development of recommendations for surgical treatment tactics for emergency surgery of severe cranio-cerebral injury. Material and methods The analysis of surgical treatment of 171 patients with severe cranio-cerebral injury who were treated at the Republican Research Center of Emergency Medicine in the period from 2018 to 2019 was performed. The criteria for inclusion of patients in the study were the presence of severe cranio-cerebral injury, the Glasgow consciousness level of less than 8 points, the presence of intracranial compressive factors (hematomas, contusion focus) requiring surgical intervention and the absence of severe combined injury, chronic hematomas, and competing somatic pathology. All patients were performed surgical treatment during the first 3-6 hours from the time of admission. Results In our work, we evaluated the results of surgical treatment according to MSCT performed in dynamics 2-3 days after the operation. The optic nerve sheath diameter was measured with a level and window width in the range of 25–300 units. The optic nerve sheath diameter was evaluated at a distance of 3 mm from the posterior contour of the eyeball before and after the surgical period. Discussion When calculating the average values in group 1, the area of skull defects was 72 cm2, in the second group - 85.7 cm2. It was associated with a lower height of the bone window for approach to the base of the skull and performing cystotomy. When measuring the bone bridge from the lower edge of the bone window to the middle of the zygomatic arch, the average value of 22 mm was noted in group 1 and 12 mm - in the second group.
Keywords:
Cranio-cerebral injury, Intracranial hematomas, Surgical technique, Basal brain tanks, Cisternotomy
Cite this paper: K. E. Makhkamov, A. B. Salaev, M. K. Makhkamov, D. U. Israilov, Features of Operative Treatment of Patients with Severe Cranio-Cerebtal Injury with the Use of Cisternotomy and Estimation of MSCT Criteria for Treatment Efficiency, American Journal of Medicine and Medical Sciences, Vol. 10 No. 8, 2020, pp. 589-596. doi: 10.5923/j.ajmms.20201008.12.
1. Introduction
The problem of treating patients with severe cranio-cerebral injury (SCCI) remains relevant in modern medicine and has great socio-economic importance. The main contingent of patients is people of the most working age up to 40-50 years [2]. The increase in disability and the costs of subsequent rehabilitation bring economic damage to any country in the world which determines the need to develop new methods of treatment and diagnostics.On average, 120, 000 victims with head injury are asked for help per year and15% of them suffering from a severe form of brain injury. Mortality remains high, varying according to various sources from 38 to 73% [1]. According to our data more than 50 thousand patients were treated due to head injury from 2001 to 2018 in the Republican Research Centre of Emergency Medicine (RRCEM), 1921 of them died. Mortality is on average about 4%, with a tendency to decrease from 6.5% to 2.2%. Neurosurgery, as one of the most high-tech specialties, with the support of the government has received significant opportunities for re-equipment in our country. Despite the improvement of the ambulance service, the introduction of modern approaches to the provision of first aid at the pre- and hospital stages, and the optimization of nosology patient routing schemes, not all patients with SCCI admit to neuro-traumatology departments. Organized visiting teams of neurosurgeons provide round-the-clock diagnostic and treatment assistance in city and district hospitals, where patients are brought from the scene. Unified approaches to the most complete and comprehensive methods of surgical treatment are of particular importance in emergency neurosurgery.Aim of the study is a comparison of the surgical treatment results of patients with severe cranio-cerebral injury and development of recommendations for surgical treatment tactics for emergency surgery of severe cranio-cerebral injury.
2. Material and Methods
The analysis of surgical treatment of 171 patients with SCCI who were treated at the Republican Research Center of Emergency Medicine from 2001 to 2018 was conducted. The criteria for inclusion of patients in the study were the presence of SCCI, the level of consciousness by the Glasgow Coma Scale (GCS) of less than 8 points, the presence of intracranial compressive factors (hematomas, contusion lesions) requiring surgical intervention and the absence of severe concomitant injury, chronic hematomas, and competing somatic pathology. All patients were performed surgical treatment during the first 3-6 hours from the time of admission. Patients were divided into 2 groups. The 1st group included 129 (75.4%) patients who underwent resection-decompressive craniotomy (RDC) with removal of the compressing substrate. The 2nd group was consisted of 42 (24.5%) who underwent RDC with opening of the basal cisterns for the purpose of additional internal decompression and early rehabilitation of the cerebrospinal fluid. Primary diagnosis was based on clinical and neurological data and the results of multispiral computed tomography. Clinical, radiological and preoperative characteristics are presented in the Table 1.Table 1. Clinical, radiological and preoperative characteristics
 |
| |
|
3. Results
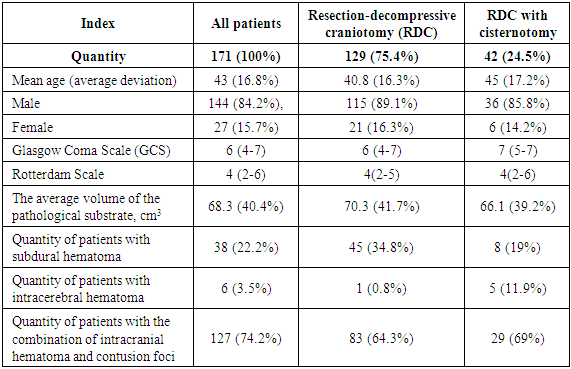
Currently, according to the international recommendations of the Brain Trauma Foundation [4], it is recommended to monitor intracranial pressure (ICP) continuously. The high cost and lack of equipment for measuring ICP in non-specialized hospitals made the team of our centre look for new approaches to assessing and treating this category of patients. In our work, we evaluated the results of surgical treatment according to MSCT data performed in dynamics 2-3 days after the surgery. The area of the postoperative skull defect and the size of the bone bridge from the lower edge of the bone window to the base of the skull in the projection of the middle of the zygomatic arch were compared.As far as basal tanks are cerebrospinal circulation pathways and contain cranial nerves and the most important vessels of the Willis circle, their compression is one of the most important indicators of the growing threat to the patient's life and level of wakefulness. We evaluated the size of the maximum width of the bypass tank in the postoperative period. The intraorbital sections of the optic nerves are surrounded by the hard and arachnoid membranes and communicate with the subarachnoid space of the brain; the increase in ICP is transmitted to the subshell space of the optic nerve through increased cerebrospinal fluid pressure, which leads to its expansion and an increase of optic nerve sheath diameter (ONSD). Clinical studies showed that ONSD increases within a few minutes following an increase of ICP and reaches its maximum value with an ICP of 35–45 mm Hg. ONSD measurement is a non-invasive diagnostic method for intracranial hypertension (ICH) and can be used as an additional criterion when deciding on further treatment tactics [5,8]. ONSD measurement was carried out with a window level and width in the range of 25–300 units. The ONSD value was estimated at a distance of 3 mm from the posterior contour of the eyeball before and after the surgical period. Examples of indicators calculation by CT data are shown in Fig. 1-4.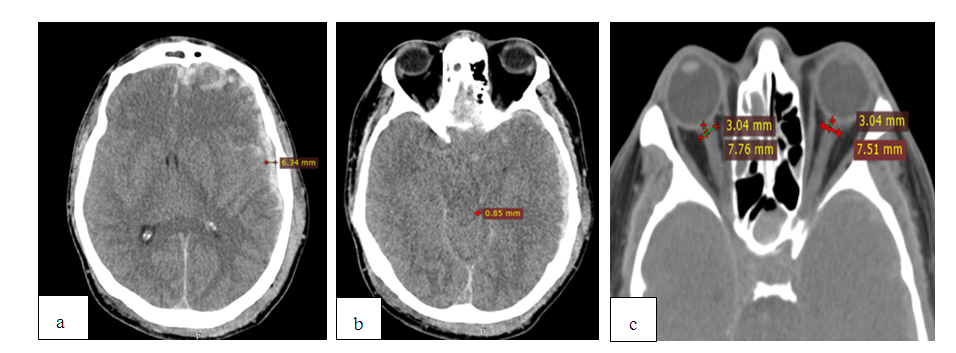 | Figure 1. MSCT before surgery: measurement of hematoma thickness (а); absence of bypass tank (b); ONSD measurement from both sides (с) |
 | Figure 2. MSCT after the same patient’s RDC a) ischemia, prolapse and impingement in the postoperative bone defect of the skull; b) maintaining the pressure of the bypass tank; c) increase of ONSD up to 8.2mm; d) Reconstruction of the postoperative bone defect of the skull, calculation of the bone bridge (39 mm) |
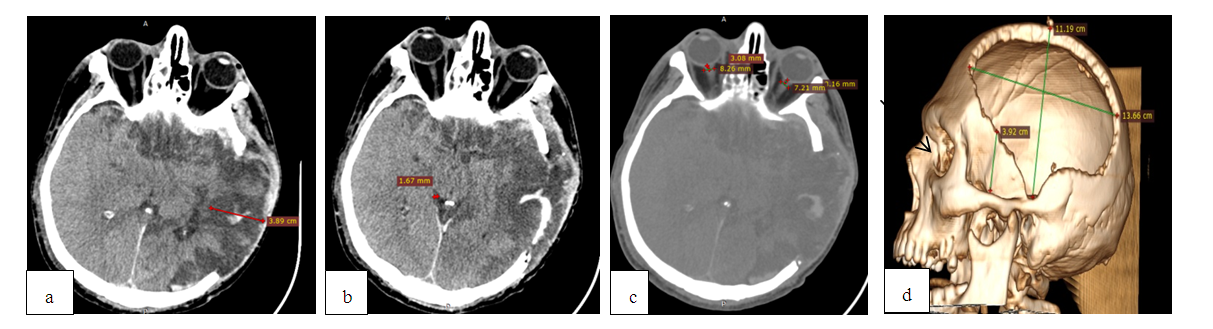
 | Figure 3. MSCT before surgery: a) a subdural hematoma with a thickness of 9.6 mm on the left; b) narrowing and deformation of the bypass tank; c) ONSD sizes up to 7.6 mm on the left |
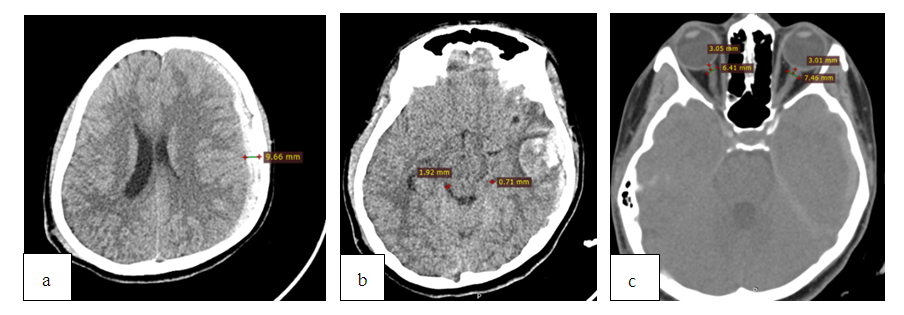
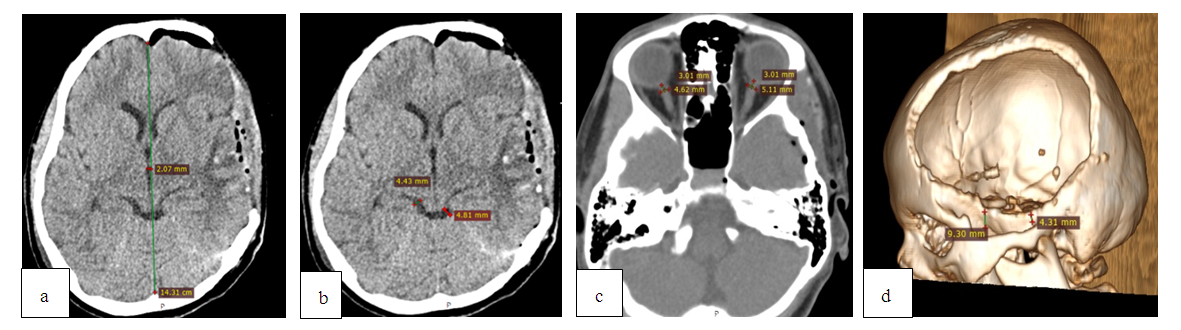 | Figure 4. MSCT after RDC with cisternotomy: a) regression of the dislocation syndrome; b) recovery of the bypass tank; c) reduction of ONSD up to 5.1 mm on the left; d) reconstruction of a postoperative skull defect; a bone bridge length is 9.3 mm |
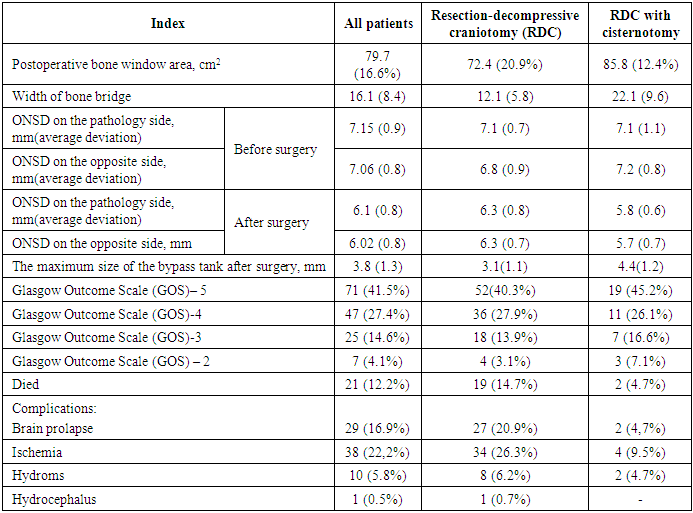
Comparative results of MSCT and postoperative outcomes are presented in Table 2.Table 2. Characteristics of radiological and postoperative outcomes
 |
| |
|
4. Discussions
When calculating the average values in group 1, the area of skull defects was 72 cm2, in the second group - 85.8 cm2. It was associated with a lower height of the bone window for the approach to the base of the skull and cystotomy. When measuring the bone bridge from the lower edge of the bone window to the middle of the zygomatic arch, the average value of 22 mm was noted in group 1 and 12 mm – in the second group. The technique of conducting RDC is different in different clinics: it depends on the availability of equipment and instruments in the hospital, the experience and knowledge of neurosurgeons, the presence of magnifying equipment (intraoperative microscope, binocular loupes). The lack of clear indications for RDC [3] in modern manuals is associated primarily with the persistent high mortality and complications. The lack of a unified approach to the conduct and technique of RDC leads to different results (insufficiency of the bone window, differences in the height of the lower edge of the bone defect, the development of postoperative brain prolapse, secondary ischemia as a result of compression in the bone window, etc.). The formation of a bone window closer to the base of the skull [10] allows to eliminate the compression of the veins of the Silvian fissure, eliminating the obstruction to the outflow of venous blood and maintaining high ICP in the postoperative period. Based on studies showing that the formation of cerebral edema (CE) is associated with the entry of cerebrospinal fluid (CSF) into the brain parenchyma through low-resistance Virchow-Robin spaces (VRS) [7] and the data that, after an injury, the glymphatic removal of excess interstitial fluid decreases [6], allowing CSF to move from brain tanks to the brain after a cranio-cerebral injury, we tried to solve problems of faster resolution of postoperative edema, early rehabilitation of cerebrospinal fluid spaces, the creation of an additional decompressive effect and early restoration of intracranial gradient ratios. Recovery and a larger increase of the bypass tank in the postoperative period by 1.4 on average, in our opinion, is associated with a microsurgical opening of the basal tanks, creating additional internal decompression of the brain [9]. The use of cisternotomy in RDC changes the quality and level of intervention, introduces a microsurgical approach to the treatment of severe cranio-cerebral injury. An increase of ONDC at an increase of ICP is associated with structural features of the optic nerve. With an increase in ICP against the background of depletion of spatial compensation mechanisms, there is a redistribution of cerebrospinal fluid from intracranial to extracranial spaces, which is accompanied by stretching of the membranes of the optic nerve and an increase of ONDC. These changes are most evident in the distal third of the optic nerve, closer to the eyeball [5].When measuring ONDC in the preoperative period in both groups, the average size was 7.1 mm. In the postoperative period there is a decrease in the average value up to 6.3 mm in the first group and in the second group of patients with cisternotomy - up to 5.7 mm, which indicates a greater decrease of ICP. Normal RDC values in this area according to CT data were calculated during a study in 300 patients without clinical and radiological signs of intracranial hypertension (ICH). According to the results of the study, ONDC values ranged from 4.94 ± 1.51mm to 5.17 ± 1.34 mm [11]. As MSCT data allow us to judge the state about the brain state in a specific period of time, the process of continuing to decrease or increase ONDC, therefore, the dynamics of ICP can be traced in a series of dynamic MSCT studies. The choice of surgical treatment method in an emergency order is determined by the neurosurgeon on duty according to the treatment standards adopted by the hospital. A significant role is played by the experience of a surgeon, possession of certain skills, as well as equipping the hospital with diagnostic equipment and intraoperative instruments. Better results with a decrease in mortality and complications obtained in our study lead to the conclusion that it is necessary to standardize surgical intervention in emergency cases. Surgical treatment should be as complete as possible, including microsurgical interventions with minimizing the risk of hematomas recurrence, infringement of the brain substance in the bone window.Based on the literature and our experience, the following recommendations are proposed for performing an unilateral RDC in an emergency:1. The patient is positioned on the operating table on his back with an elevated head end of 25-30 degrees, turning his head in the contralateral direction. The head is fixed with a head holder and a roller under the shoulder on the side of the operation, which eliminates sharp movements of the patient’s head when performing the bony part of the trepanation and excessive bending of the neck, which lead to a violation of the venous outflow and aggravation of cerebral edema.Before cutting the skin, to reduce bleeding from multiple small vessels of the skin and subcutaneous tissue, infiltration is used along the cut line of the vasoconstrictor solution with a vasoconstrictor target (Adrenaline solution 0.18% -1ml, Sodium chloride 0.9% -100ml, Lidocaine solution 2% -4ml ) (Fig. 5). | Figure 5. Introduction of a vasoconstrictor along the skin incision line |
2. A standard cut is performed according to the type of question mark - traumaflap. A skin incision begins 1 cm in front of the tragus at the level of the zygomatic process. Then continue up and slightly in front of the ear. Further, posteriorly towards the parietal-occipital region, bend around the parietal tubercle and follow parasagittally to the frontal region, not reaching 2–2.5 cm to the midline and in front, to the border of the scalp (Fig. 6.). | Figure 6. Marking the cut line by type of question mark (traumaflap) |
3. The branch of the superficial temporal artery and vein must be preserved by taking it on the holders. Subsequently, the skin is cut to the temporal muscle, which is then dissected by an electric knife. The formed flap is folded back in one block (Fig. 7).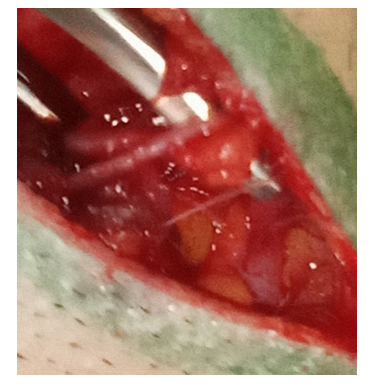 | Figure 7. Preservation of the branches of the superficial temporal artery |
4. The application of milling openings and cutting of the bone flap is traditionally carried out - after detachment of the periosteum, several milling openings are applied and the bone is resected using Luer pliers or a craniotome within: in front - to the level of the lateral border of the orbit, in the back - up to 4 cm posterior to the external auditory opening, from above - to the level of the superior sagittal sinus, from below - to the level of the middle cranial fossa, performing temporal decompression, thereby freeing the pole of the temporal lobe. After cutting the bone flap along its perimeter and the perimeter of the bone window, several holes with a diameter of 2-3 mm are sawed with a drill for subsequent suturing of the dura mater (DM) in order to prevent postoperative epidural hematomas. In case of damage to the basal parts of the frontal and temporal lobe, an additional pterioneal approach is performed with resection of the wing of the main bone, until a single plane is reached with the lateral part of the anterior cranial fossa. This creates a larger overview in the surgical wound and the possibility of microsurgical manipulations; decompression of the projection of the Silvian’s fissure and its anatomical structures is achieved (Fig. 8).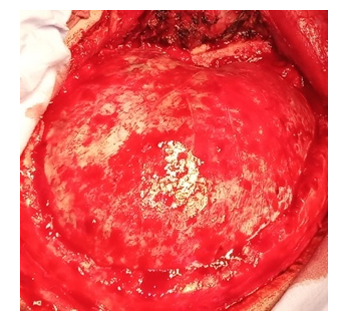 | Figure 8. Formation of a skull defect with the resection of the main bone wing |
5. Autopsy DM should prevent injury of the underlying cortex at severe cerebral edema. To do this, the scalpel’s blade is directed tangentially to the surface of the outer DM leaf, the outer leaf is grabbed with the tip of the scalpel and lifted while grasping it with tweezers, while local conditions of decompression under the shell are created, where the cerebrospinal fluid rises, creating protection of the brain from injury when opening the inner DM leaf. The shell is opened at a distance, retreating more than the thickness of the bone from the edge of the defect, in order to avoid injury of the cortex by the edge of the defect in cases of the brain prolapse (Fig. 9).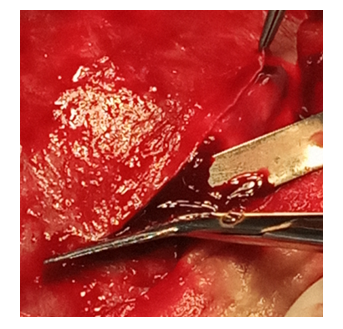 | Figure 9. Autopsy of DM, indent from the edge of the bone defect to the thickness of the bone |
6. Removal of hematoma. Blood clots are removed by washing with a stream of physiological saline and electric suction pumps around the trepanation window. You cannot manipulate the suction “blindly” beyond its edges. It can trigger bleeding from the cortical arteries or transitional veins. At this stage, it is mandatory to use a binocular magnifier with an illuminator or an operating microscope which allows you to revise the pole of the frontal lobe, the basal parts of the temporal and frontal lobes (Fig. 10).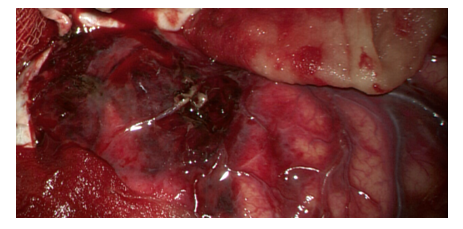 | Figure 10. View of the injured brain with contusion foci |
7. Sanitation of the brain crushing centers. Clearly non-viable areas of crushing of the brain substance in the form of brain detritus and blood clots, causing an additional mass effect and zones of perifocal edema are removed (Fig. 11).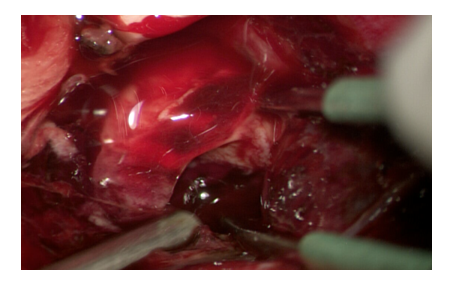 | Figure 11. Sanitation of contusion foci with removal of intracerebral hematoma |
8. At multiple contusion foci, massive subarachnoid hemorrhage, lingering brain pulsation, a cisternotomy is performed with the aim of sanitizing the cerebrospinal fluid from the basal cisterns, additional internal decompression. Approach to the base of the skull is carried out by the “tweezers and suction” method, by traction of the frontal lobe pole. Subsequently, the optic nerve is visualized from the approach side, an opto-chiasmal cistern is opened (Fig. 12).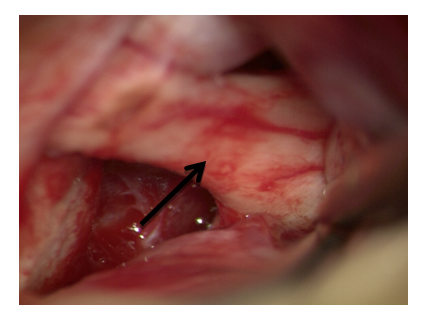 | Figure 12. Approach to the optic nerve by the “tweezers and suction” method |
Next, the optical-carotid cistern is opened by the method of acute dissection with blunt-pointed microscissors, which reduces the risk of non-contact rupture of blood vessels (Fig. 13).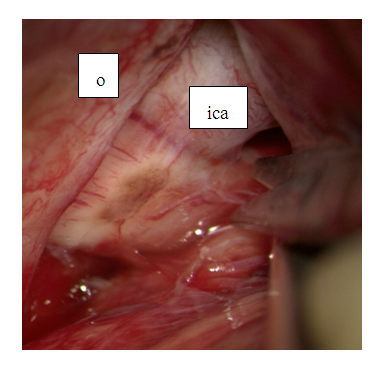 | Figure 13. Dissection of the optic-carotid cistern: o-optic nerve, ica – internal carotid artery |
The dissection of the optic-carotid cistern is carried out from the medial part of the internal carotid artery and the lateral part of the optic nerve because there are no perforating arteries there. Dissection is continued in the distal direction to the proximal part of the Sylvian fissure. Subsequently, the opening of the intersternal cistern, i.e. Liliquist membranes is performed (Fig. 14).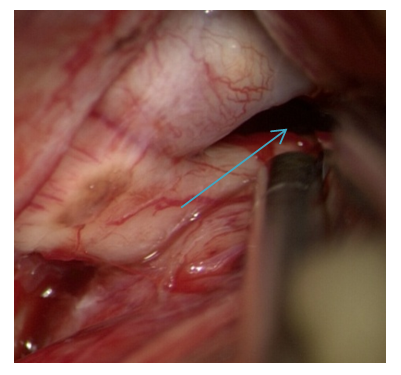 | Figure 14. Lilquist membrane is opened |
The course of surgical approach is directed to the projection of the prechiasmal cistern, which is subsequently opened by acute dissection (Fig. 15).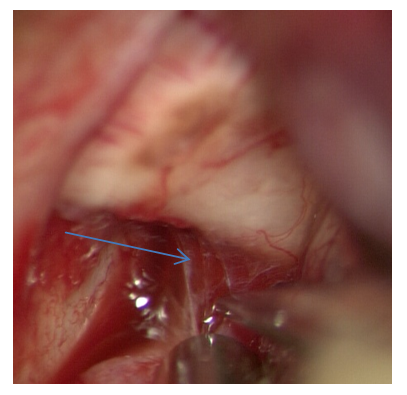 | Figure 15. Dissection of the prechiasmal cistern |
The chiasm, the terminal plate is visualized, then the terminal plate is opened (Fig. 16).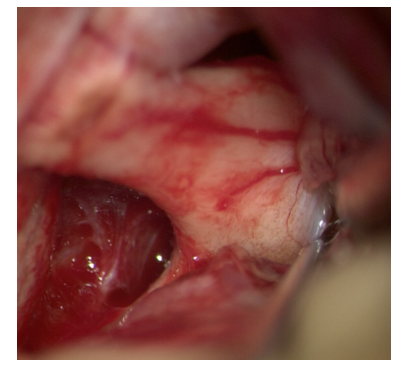 | Figure 16. Opening of the terminal plate |
9. After opening all of the above mentioned cisterns of the brain, relaxation of the brain substance is achieved with the appearance of a satisfactory pulsation. With the development of intraoperative malignant cerebral edema, it must act strictly sequentially, panic wound closure is unacceptable. It is necessary to quickly assess the adequacy of the bone window, taking into account the situation, if necessary, rapid expansion along the perimeter, additionally resection of the temporal muscle, removal of contusion foci and low-functional areas of the brain (anterior thirds of the middle and lower temporal convolutions [10], opening of the pre- and chiasmal cisterns until the cerebrospinal fluid appears.10. Particular attention should be paid to hemostasis. In the acute period of trauma due to reactive hyperemia, the tissues bleed more intensively, the brain substance is more vulnerable against the background of edema. In addition, all patients with acute trauma have bleeding disorders and relapses or postoperative hematomas are one of the leading causes of adverse outcomes. Traction of the brain, sharp and gross manipulations in the wound should be avoided. special attention should be paid to the degree of dilution of aspiration - its excessiveness leads to a relapse of bleeding from previously thrombosed, or mechanical damage of intact vessels. In the case of bleeding, it is necessary to clearly visualize the bleeding vessel by irrigation of the wound or by temporary pressing of wet cotton swabs and coagulate it, starting with a small current strength. At diffuse bleeding, coagulation, which can further provoke it, should be avoided. It is better to use a hemostatic absorbable cheesecloth "Surzhitsel" or the drug "Tachocomb". At the end of the operation, the dura mater is sutured with an additional patch from the aponeurosis of the temporal muscle or periosteum, while this creates an additional reserve space for the circulation of cerebrospinal fluid, the implementation of postoperative cerebral edema without disturbing cerebrospinal fluid and hemodynamics. Adequate drainage of the wound is necessary by the end of the surgery. The use of rubber strips for this purpose is often ineffective. It is better to use soft, thin silicone tubes that are left in the bed of the removed intracranial hematoma or in the projection of the brain cisterns, which allows additional sanitization of the cerebrospinal fluid and reduce intracranial hypertension.
5. Conclusions
Under the conditions of the Republican Research Centre of Emergency Medicine, the surgical treatment of patients with SCCI should be complete and exclude the most frequent complications in the form of postoperative edema, prolapse, ischemia, hematoma recurrence, maintaining high ICP. The use of ONDC supplemented by pterional approach, cisternotomy of basal cisterns is the most consistent with these goals.
References
| [1] | Struk Yu.V. et al. "The provision of emergency neurosurgical care" Journal named after. N.V. Sklifosovskiy "Emergency medical care" - No. 3, 2015, P. 36. |
| [2] | Makhkamov K.E., Salaev A.B. “Methods of surgical treatment of severe traumatic brain injury” Bulletin of emergency medicine 2018 Vol. 11 No.4, P. 73. |
| [3] | Сarney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury, 4th ed. Neurosurgery. 2016; 80(1): 6-15. https://doi.org/10.1227/NEU.0000000000001432. |
| [4] | Helmke K, Hansen HC. Fundamentals of transorbitalsonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patientstudy. Pediatr Radiol. 1996; 26: 706-710. |
| [5] | Launey Y, Nesseler N, Le Maguet P, Mallédant Y, Seguin P. Effect of osmotherapy on optic nerve sheath diameter in patients with increased intracranial pressure. J Neurotrauma. 2014; 31: 984-988. https://doi.org/10.1089/neu.2012.2829. |
| [6] | Bulloсk R.М., Chesnut R.M., Clifton G.L. Management and prognosis of severe traumatic brain injury // Brain Trauma Foundation (c), Vashington, 2000. — 286 р. |
| [7] | Puras Y.V.,. Talypov A.E., Khovrin D.V., Krylov V.V. "Selective microsurgical resection of the temporal lobe in the surgical treatment of the dislocation syndrome of patients with severe traumatic brain injury" Journal of Neurosurgery, № 2, 2012. p 46. |
| [8] | Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014; 34: 16180–93. |
| [9] | Cherian I, Bernardo A, Grasso G. Cisternostomy for traumatic brain injury: Pathophysiological mechanisms and surgical technical notes. World Neurosurg. 2016; 29: 51–7. |
| [10] | Makhamov K.E., Salaev A.B. Estimation of results and microsurgical aspect at severe craniocerebral injury American Journal of Medicine and Medical Sciences 2019, 9(10): 365-371. DOI: 10.5923/j.ajmms.20190910.03. |
| [11] | Vaiman M, Abuita R, Bekerman I. Optic nerve sheath diameters in healthy adults measured by computer tomography. Int J Ophthalmol. 2015; 8: 1240- 1244. https://doi.org/10.3980/j.issn.2222-3959.2015.06.30. |
















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


