-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(7): 525-527
doi:10.5923/j.ajmms.20201007.17

Study of the Prebiotic Activity of Inulin and Fructooligosaccharides Allocated from Helianthus Tuberosus in Experiences in Vivo
Jannat I. Islamova
Institute of Chemistry of Plant Substances named after Academician S. Yu. Yunusov, Academy of Sciences of the Republic of Uzbekistan
Correspondence to: Jannat I. Islamova, Institute of Chemistry of Plant Substances named after Academician S. Yu. Yunusov, Academy of Sciences of the Republic of Uzbekistan.
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In in vivo experiments, the prebiotic activity of inulin and fructooligosaccharides isolated from Jerusalem artichoke (Helianthus tuberosus L., family Asteraceae) was studied. It was found that the studied substances have a pronounced prebiotic effect, while the most effective effect was found in fructooligosaccharides.
Keywords: Jerusalem artichoke, Inulin, Fructooligosaccharides, Prebiotic activity, In vivo experiments
Cite this paper: Jannat I. Islamova, Study of the Prebiotic Activity of Inulin and Fructooligosaccharides Allocated from Helianthus Tuberosus in Experiences in Vivo, American Journal of Medicine and Medical Sciences, Vol. 10 No. 7, 2020, pp. 525-527. doi: 10.5923/j.ajmms.20201007.17.
- The human gastrointestinal tract is a complex symbiotic ecosystem, formed during the long joint evolution of humans and microorganisms. During a person’s life, both the ratio and the number of microorganism cells can change for a number of reasons. Transient disorders of intestinal microbiocenosis are referred to as dysbacterial reactions, and pronounced and persistent changes in the quantitative and qualitative composition of microorganisms are referred to as dysbacterioses [1,2]. The use of prebiotics (bifidus factors) - non-microbial preparations that can resist gastric acidity, hydrolysis by mammalian enzymes and absorption in the upper gastrointestinal tract, which are selectively fermented food ingredients and can have a positive effect on the host through selective growth stimulation or enhancing the metabolic activity of normal intestinal microflora [3], allows to solve the problem of treatment of dysbiosis.In this work, we studied inulin and fructooligosaccharides (FOS) isolated from Jerusalem artichoke (Helianthus tuberosus L., family Asteraceae) as potential prebiotic agents. The tubers of this plant are famous for their therapeutic effects in non-insulin-dependent diabetes mellitus in combination with obesity; they also note an improvement in the state of the vascular wall and blood rheological parameters. Jerusalem artichoke has a choleretic effect, normalizes the functional state of the liver, and increases the nonspecific resistance of the body [4]. To confirm the data on the high prebiotic activity of inulin and FOS, which we obtained earlier in in vitro experiments [5], we studied their effectiveness in a series of in vivo experiments - correction of intestinal microbiocenosis disturbances in experimental animals with antibiotic therapy with gentamicin.
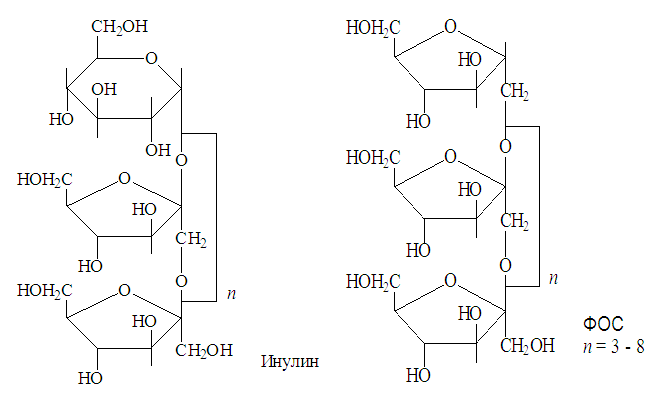 | Figure 1. Structural formulas of inulin and fructooligosaccharide (FOS) |
1. Materials and Methods
- Inulin and fructo-oligosaccharides (FOS) from Jerusalem artichoke tubers were obtained, as described in [6], in the laboratory of the chemistry of high-molecular compounds IHRV AN RUz. Inulin is a fructose polysaccharide (polyfructosan) is a product of photosynthesis of some plants and is a mixture of structurally similar polymer analogs containing about 35 fructose residues, with a variable specific rotation [α] D from 32.0 to 40.0. Inulin yield 16% by weight of air-dry raw materials. The molecular weight is 5000-6000 Da. Slightly soluble in cold water, but soluble in hot water. Inulin is used in the manufacture of many pharmaceuticals.FOS - dry absorbent powder, light brown, readily soluble in water, sweetish taste. The output of FOS is 30% of air-dry raw materials.The experiments used white outbred mice of both sexes weighing 18-20 g. The development of microbiocenosis disorders in them was initiated by the administration of the drug gentamicin (KRKA, Slovenia) using a special probe in the stomach at a dose of 2.9 mg / kg twice a day for 7 days [7]. Presented in the work of B.A. Shenderova (1998), the data indicate that when a number of aminoglycosides are orally administered, the concentration of the latter in 1 g of human feces can reach 20,000-24,000 μg, which significantly exceeds the minimum inhibitory concentration for most bacteria of fecal microflora. Gentamicin, unlike other aminoglycosides, when ingested is practically not absorbed in the gastrointestinal tract and has a local effect. Causing microecological disturbances in the intestine, gentamicin initiates the development of intestinal dysbiosis in experimental laboratory animals when administered orally. The studied compounds and the comparison drug, lactulose, were also administered orally, starting from the moment of administration and throughout the week the animals received gentamicin in doses of 100.0 mg / kg.From the first day of the administration of the antibiotic gentamicin, as well as against the background of the administration of inulin, FOS and the drug for comparing lactulose, animals took feces to determine the amount of bifidobacteria and lactobacilli.To carry out crops on nutrient media, weighed up to 1 g of material. Sterile saline was added to the sample at a rate of 1:10 (weight / volume). The material was thoroughly homogenized with a glass rod and left at room temperature for 10-15 minutes. From the prepared dilutions, metered inoculations were made on culture media and all material was thoroughly triturated over the entire surface of the agar with sterile spatulas to obtain the growth of isolated colonies of cultured microorganisms available for counting on agarized media. All media were incubated under appropriate conditions. Accounting for the results of the study included identification of the species and determination of the number of microorganisms [8]. For this purpose, after incubation, bacterial colonies were counted on solid nutrient media, taking into account the degree of feces dilution and the sowing dose according to the formula: M = N x 10n + 1, where M is the number of microorganisms in 1 g of feces, N is the number of colonies grown in a dish, n - the degree of breeding feces.Identification of microorganisms was carried out by the characteristic cultural properties on agar media, as well as (mandatory for bifidobacteria and lactobacilli) by the presence of characteristic cells in Gram stained smears. Bifidobacteria give a characteristic growth of colonies — gram-positive rods in the form of hieroglyphs, cell sizes from 4 to 10 μm, with thickened “clubs” or bifurcated ends. Lactobacilli are characterized by colonies 1-3 mm in size, transparent, convex with even or indented edges, lenticular or star-shaped, which in the smear have the form of gram-positive rods arranged in short chains (according to Hoult J. et al. 1997).The experiments were carried out in five replicates. Statistical processing of the experimental results was carried out in accordance with the recommendations set out in the I.P. Ashmarina and A.A. Vorobyov (1962).
2. Results and Discussion
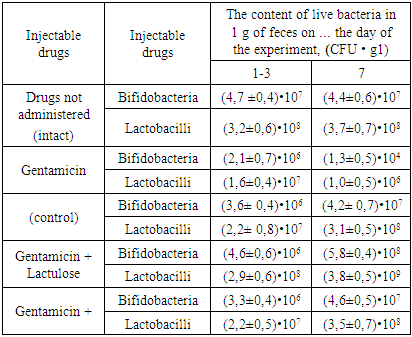
- Dysbiotic changes in the composition of the intestinal microflora began to appear already at the beginning of the experiment (1-3 days) under the influence of oral administration of gentamicin, which was confirmed by the results of bacteriological studies of feces in experimental animals. So, if the number of bifidobacteria and lactobacilli in intact mice was (4.7 ± 0.4) • 107 and (3.2 ± 0.6) • 108 CFU • g1, respectively, then in mice their content was (2, 1 ± 0.7) • 106 and (1.6 ± 0.4) • 107 CFU • g1. At the same time, visually dysbiotic changes in animals began to manifest themselves as hypokinesia and loose stools. At the same initial stage, against the background of developing dysbiosis, the positive effect of inulin and FOS, more pronounced than that of lactulose, on the number of viable bifidobacteria and lactobacilli contained in the feces of animals of the experimental groups was established, with FOS being more effective than inulin. Similar results were obtained in in vitro experiments.On the 7th day after the start of the experiment, the animals in each of the groups again selected feces for bacteriological studies. In the control group receiving only gentamicin, there was a significant decrease in the number of bifidobacteria - (1.3 ± 0.5) • 104 and lactobacilli - (1.0 ± 0.5) • 106. The marked pronounced dysbiotic changes in the intestinal microflora were now accompanied by obvious violations of the general condition of the animals, consisting in external untidiness, decreased activity, poor eating of food, and the excretion of light-colored liquid feces. In the experimental groups, there was an increase in the number of these microorganisms in terms of 1 g of feces against the background of the introduction of FOS (bifidobacteria- (5.8 ± 0.4) • 108, lactobacilli - (3.8 ± 0.5) • 109) and inulin (bifidobacteria- (4.6 ± 0.5) • 107, lactobacilli - (3.5 ± 0.7) • 108), exceeding the values obtained with the introduction of lactulose (bifidobacteria- (4.2 ± 0.7) ) • 107, lactobacilli - (3.1 ± 0.5) • 108) and even the values of the intact group (bifidobacteria- (4.4 ± 0.6) • 107 and lactobacilli - (3.7 ± 0.7) • 108). The animals of these groups were the exact opposite of the control: receiving the antibiotic gentamicin and FOS / inulin / lactulose, the animals remained active, mobile, neat, well-fed food.
3. Conclusions
- The obtained experimental data obtained in in vitro experiments and confirmed by the results of in vivo experiments on the correction of dysbiotic disorders in the intestinal microflora in experimental white mice with experimental antibiotic-associated dysbiosis suggest the possibility of using inulin and fructooligosaccharides as potential prebiotic agents, isolated from Jerusalem artichoke (Helianthus tuberosus L.).
References
| [1] | Ardatskaya M.D. Intestinal dysbiosis: concept, diagnosis, principles of therapeutic correction. Consilium ruedicum 2008; 10 (8): 86-92. |
| [2] | Ardatskaya M. D., Belmer S. V., Dobritsa V. P. et al. Zakharenko S. M., Lazebnik L. B., Minushkin O. N., Oreshko L. S., Sitkin S. I., Tkachenko E.I., Suvorov A.N., Khavkin A.I., Shenderov B.A. Intestinal dysbiosis (dysbacteriosis): current state. Problems, comprehensive diagnostics and therapeutic correction // Experimental and clinical gastroenterology. - 2015. -T. 117.-No.5. P.13-50. |
| [3] | Roberfroid M.B. Prеbiotics: the concept revisited // J. Nutr. – 2007. –Vol. 137, № 3. – P. 830-837P. |
| [4] | Pronchenko G.E., Vandyshev V.V. Plants - donors of dietary supplements: the pros and cons. - M.: GEOTAR –Meditsina, 2013. - P. 189-190. |
| [5] | Islamova Zh.I., D.K. Oh, K.S. Zhauynbaeva, D.A. Rakhimov, Z.A. Khushbaktova, R.K. Rakhmanberdyev, V.N. Cheeses. A comparative study of the prebiotic activity of inulin and fructooligosaccharides isolated from Jerusalem artichoke. Journal of Theoretical and Clinical Medicine. No. 1. 2016. P. 44-46. |
| [6] | Rakhimov D.A., Zhauynbaeva K.S., Mezhlumyan L.G., Syrov V.N., Khushbaktova Z.A., Salikhov S.A., Mavlyanova R.F. Carbohydrate and protein components of Helianthus tuberosus, their biological activity // Chemistry of nature. connection - 2011. - No. 4. - P.449 - 452. |
| [7] | Chicherin I.Yu., Darmov I.V., Pogorelsky I.P., Lundovskikh I.A., Gavrilov K.E. The survival of bifidobacteria and lactobacilli in vitro in the gastric juice and duodenal contents of people.// Medical Almanac 2012. - No. 1. - P.57-59. |
| [8] | Bondarenko V.M., Likhoded V.G. Mirobiological diagnosis of intestinal dysbiosis. // Methodical recommendations. Moscow. GU NIIEM them. N.F. Gamalei RAMS. - 2007. – 70p. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML