-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(7): 522-524
doi:10.5923/j.ajmms.20201007.16

Anti-Parasitic Activity of Sesquiterpene Lactones Tanacetum Pseudoachillea
Jannat I. Islamova
Institute of Chemistry of Plant Substances named after Academician S. Yu. Yunusov, Academy of Sciences of the Republic of Uzbekistan
Correspondence to: Jannat I. Islamova, Institute of Chemistry of Plant Substances named after Academician S. Yu. Yunusov, Academy of Sciences of the Republic of Uzbekistan.
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

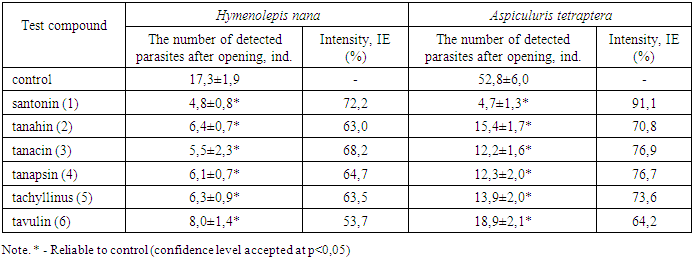
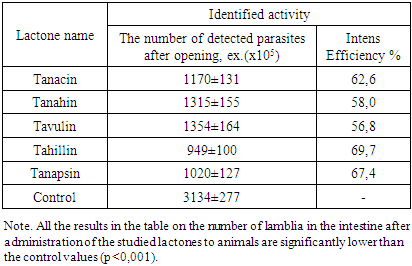
Studies have shown that the high antiparasitic activity of Tanacetum pseudoachillea is due to the presence of sesquiterpene lactones of tanahine, tanacin, tanapsin, tachyllin and tavulin. For these lactones, a more pronounced anthelmintic effect against nematodes is characteristic than cestodes and a certain antilamblious activity. The plant Tanacetum pseudoachillea can be considered as a promising source for the development of new antiparasitic agents.
Keywords: Tanacetum pseudoachillea, Sesquiterpene lactones, Antiparasitic activity
Cite this paper: Jannat I. Islamova, Anti-Parasitic Activity of Sesquiterpene Lactones Tanacetum Pseudoachillea, American Journal of Medicine and Medical Sciences, Vol. 10 No. 7, 2020, pp. 522-524. doi: 10.5923/j.ajmms.20201007.16.
1. Introduction
- Natural sesquiterpenic lactones have multifaceted biological activity [1]. Of considerable interest is the study of the antiparasitic activity of sesquiterpene lactones, since a number of effective anthelmintic (santonin, gelenin) and antimalarial (artemisinin and its derivatives) agents have already been identified [2,3]. Tanacetum pseudoachillea C. Winkl. (tansy yarrow), growing in Central Asia, is one of the promising sources of sesquiterpenic lactones. It was previously shown that Tanacetum pseudoachillea has anthelmintic properties [4].The aim of the study of this work was to determine the antiparasitic (anthelmintic and antiprotozoal) activity of the activity of individual sesquiterpene lactones of tanahine, tanacin, tanapsin, tachyllin and tavulin isolated from tansy yarrow.Materials and methods. For research, sesquiterpene lactones tanahine, tanacin, tanapsin, tachyllin and tavulin were isolated according to the known method [5] from the aerial part of Tanacetum pseudoachillea.Anthelmintic (anti-cestodose and anti-nematodosis) activity was studied in comparison with santonin on white outbred male mice weighing 18-20 g. An experimental model of cestodosis - hymenolepidosis was caused by infection of each animal at the rate of 200 invasive eggs Hymenolepis nana orally, the model of nematodosis from each animal aspurulosis the calculation of 100 invasive eggs of Aspiculuris tetraptera is also orally (mouse aspiculurosis is an adequate model of enterobiosis) [6].The studied lactones were injected into animals with an intragastric atraumatic probe at a dose of 20 mg / kg in the form of an aqueous emulsion with apricot gum. Control animals that received an adequate amount of only an aqueous emulsion of apricot gum.In mice with hymenolepidosis, the test substances were administered on the 10th day after infection (the imaginal stage of H. nana development) once a day for 5 days. On the 14th day after infection, the animals were slaughtered under light ether anesthesia, a small section of the small intestine 10 cm long was removed, it was opened and counted cestodes attached to the wall of the small intestine.In mice with apiculurosis, lactones were started on the 10th day after infection once a day for 5 days. On the 4th day after the end of the administration of lactones, the results were evaluated by extracting and counting mature nematodes from a 10 cm long section of the colon.Antiprotozoal activity was studied in experiments with experimental giardiasis using white outbred mice of both sexes weighing 13-15 g. To reproduce the experimental model, animals were infected orally with a suspension containing cysts and trophozoites of Giardia muris 5 × 103 in 0.5 ml [6]. A suspension was prepared from the contents of the small intestine of spontaneously infected mice (Roberts-Thomson, Mitchell, 1978). The studied lactones were injected into mice with a special atraumatic probe into the stomach in the form of an aqueous emulsion with gum arabic at the rate of 20 mg / kg on the 5th day after infection for the next 5 days. All experiments were carried out in accordance with the requirements of the "European Convention for the Protection of Vertebrate Animals Used for Experiments or for Other Scientific Purposes" (Strasbourg, 1986). After treatment, the mice were sacrificed under mild ether anesthesia by cervical dislocation and 10 cm of the small intestine were taken, starting from the gastroduodenal articulation, from each animal. Each segment was opened in the longitudinal direction in 5 ml of physiological saline, placed on ice for 10 min, and mixed on a vortex mixer to disconnect trophozoites from the mucosa. The intestinal contents were washed with isotonic solution (0.85%) of sodium chloride (NaCl) by repeated centrifugation (3 min × 1500 rpm) until a clear supernatant was formed, which was carefully removed by pipetting. A flotation solution (saturated (30%) sucrose solution (18–20°C)) was added to the remaining faecal sediment with a volume 3–4 times larger than the faecal volume with vigorous stirring. The tubes were centrifuged at 1500 rpm for 5 minutes. After a 10-15 minute exposure, the surface film of the flotant (supernatant) was carefully removed with a 0.1 ml pipette dispenser and transferred to a test tube with an isotonic sodium chloride solution (1 ml). The procedure is repeated at least 3 times. Using a hemocytometer, the total number of trophozoites and lamblia cysts was counted in suspension. The effectiveness of the studied lactones was judged by calculating the average number of vegetative forms and lamblia cysts per animal.The effectiveness of sesquiterpene lactones was determined by calculating the average number of detected parasites and intensity efficiency (IE), to calculate which the average number of parasites in opened animals in the experimental and control groups was compared and determined (in%) by the formula: IE = 100 (K-O) / K, where K is the average number of helminths in the control group; O-average number of helminths in the experimental group [6].The results obtained were processed by the method of variation statistics using the Student t-test.
2. Results and Discussion
- Previous phytochemical studies showed that sesquiterpene lactones tanahine (2), tanacin (3), tanapsin (4), tachyllin (5) and tavulin (6), isolated from the aerial part of Tanacetum pseudoachillea, belong to two structural skeletons types: germacranolides (2, 3, 6) and evdesmanolides (4, 5) (Fig. 1).
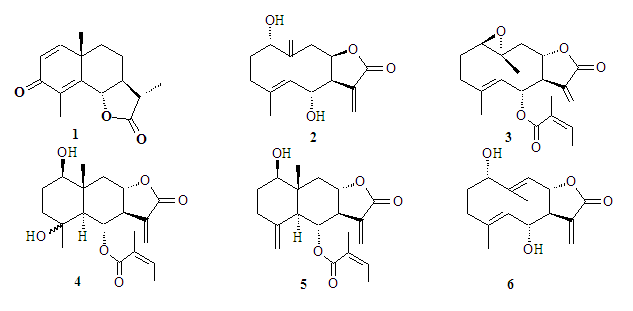 | Figure 1. Structures of sesquiterpene lactones Tanacetum pseudoachillea |
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML