-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(7): 515-521
doi:10.5923/j.ajmms.20201007.15

The Role of Supportive Computer Program in Diagnosis, Management, Prevention and Prognosis of Fat Embolism Syndrome Following Skeletal Trauma
Khadjibaev A. M., Valiev E. Y., Mirdjalilov F. H., Alimov A. H., Khakimov R. N.
Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article discusses the current state of the problem of fat embolism. The role of supportive computer program in the diagnosis, prevention and prognosis of fat embolism syndrome is discussed, as well as the effectiveness of various types of osteosynthesis at the stages of treatment of patients with fractures of tubular bones. A case study is given as an example.
Keywords: Fat embolism syndrome, Fat globulemia, Combined trauma
Cite this paper: Khadjibaev A. M., Valiev E. Y., Mirdjalilov F. H., Alimov A. H., Khakimov R. N., The Role of Supportive Computer Program in Diagnosis, Management, Prevention and Prognosis of Fat Embolism Syndrome Following Skeletal Trauma, American Journal of Medicine and Medical Sciences, Vol. 10 No. 7, 2020, pp. 515-521. doi: 10.5923/j.ajmms.20201007.15.
- 15 to 35% of severe combined trauma patients die due to severe complications of trauma. One of the life threatening complications of trauma is fat embolism syndrome (FES) [4,11,12,17].Fat embolism syndrome (FES) is defined as a clinical condition characterized by dysfunction of the lungs and central nervous system due to microvascular obstruction with large globules of fat, occurring mainly after fractures of tubular bones or pelvis [6,8,9,18,19].Despite the progress achieved in the treatment of multiple and combined injuries, the problem of early diagnosis of this syndrome remains unsolved, especially at the stage of preclinical manifestations. A wide range of studies has been proposed for the diagnosis of FES. However, none of them has 100% specificity. The existing laboratory and instrumental methods of diagnosing FES are not satisfactory and changes are mainly detected when clinical picture is expanded and usually they only confirm the clinical diagnosis [3,5,7,15].Diagnosis of FES remains a difficult and not fully resolved problem, because of absence of clear clinical picture and pathognomonic symptoms. Specific and highly informative laboratory tests are yet to be developed. At the same time, the clinical manifestations of FES in severe combined trauma can be blinded by a picture of traumatic shock, severe traumatic brain injury (TBI) and post-traumatic respiratory failure of different etiology. There is no single rational scheme for the prevention and treatment of FES [1,13,14,16].We aimed to optimize methods of diagnosing, predicting, preventing and treating of FES in patients with fractures of long bones and pelvis by creating a pathophysiology based scoring system with objective criteria.
1. Material and Methods
- We studied the patterns of occurrence of FES in patients with skeletal trauma. Retrospective analysis of medical records of 181 patients with diagnosis of fat embolism syndrome treated in the Trauma Department of the Republican Research Centre of Emergency Medicine of Uzbekistan in the period of 2010 to 2017 was performed. 124 (68.5%) of patients were males. The largest number of patients 65.2% were young, most of working age. According to the mechanism of injury 158 (87.2%) cases were results of motor vehicle accidents. 131 (72.3%) patients were diagnosed with traumatic shock upon admission, 43 (23.7%) patients were in a state of alcohol intoxication. The scope of diagnosis and care is strictly regulated by the diagnostic and treatment standards developed in the clinic.In addition, 155 patients (85%) had TBI, 16 patients (8.8%) had combined abdominal trauma, 66 patients (36.4%) had chest trauma, 32 patients (17.6%) had facial trauma, 9 patients (4.9%) hadinjuries of main arteries of lower extremities.Mostly, the clinical picture of the FES developed after the "light gap" from 12 to 72 hours. 23 patients (12.8%) developed an acute form of FES, in 44 patients (23.8%) and in 112 patients (63.4%) subacute and subclinical forms of fat embolism were observed respectively. The mixed clinical form prevailed in 94 patients (52%), pulmonaryformin 67 (37%) and cerebral form occurred in 20 (11%) patients.
2. Results and Discussion
- For the diagnosis of FES we used a set of large and small diagnostic criteria published by Gurd [2]. Major signs include: respiratory failure; cerebral involvement; petechial rash. Minor signs are hyperthermia; tachycardia; retinal changes; jaundice; renal signs and laboratory confirmation of the diagnosis: fat globulemia; anemia; thrombocytopenia; red blood cell segmentation. At least two positive major criteria plus one minor criterion or four positive minor criteria with the obligatory identification of globulemia are suggestive of FES diagnosis.In accordance with the above diagnostic scheme, we analyzed the frequency of major andminor criteria and laboratory signs. The following data was obtained:- respiratory failure (tachypnea, dyspnea and with or without cyanosis, a decrease in PaO2 and an increase in PaCO2) - occurred in 85.1% of patients with FES;- braindysfunction not associated with TBI (disorientation, drowsiness, lethargy, convulsions, coma) - observed in 86% of cases;- petechial rash on mucous membranes and skin of the chest and neck, was noted in 76.6% of patients.In our study 33.8% of patients developed major signs within 24 hours and 66.2% within 48 hours.Also, studying the frequency of occurrence of minor signs, the following results were obtained: fever in 74.3% of cases, tachycardia in 97.2%, retinal changes in 67.9%, jaundice in 31.5% and symptoms of kidney injuryin 46.1% cases.Laboratory data (day 1): anemia was observed in 76.9% of patients, thrombocytopenia in 57.8% of cases, an increase in erythrocyte sedimentation rate (ESR) of 45.1%, and fat globulemia in 96.3% of cases.According to the results of the analysis, we developed a quantitative assessment of symptoms significance with the calculation of fat embolism score index, where petechial rash and diffuse alveolar infiltration rated with highest scores, and hypoxemia, confusion, hyperthermia, tachycardia and tachypnea with gradually decreasing significance. All the most frequently detected symptoms of FES were systematized, taking into account their specificity which was given a point value. In addition, to data such as number of damaged musculoskeletal system segments, the adequacy of their immobilization at the prehospital stage, the severity of shock, severity of blood loss etc. were included (Table 1).
|
3. Clinical Case
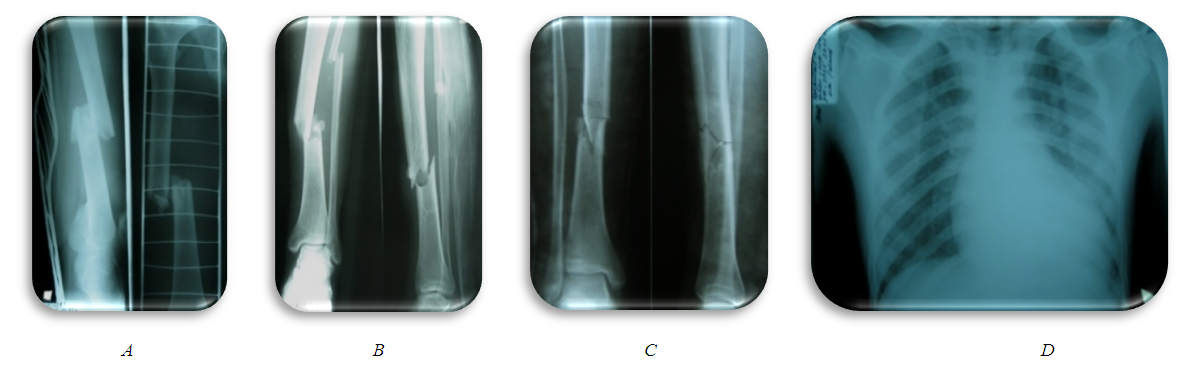
- 25 y/o male admitted to Emergency department (ED) after motor vehicle accident. He was a pedestrian, delivered 45 minutes after injury. The whole diagnostic measures was carried out in ED according to the standard (Fig. 1). Examination of traumatologist, neurosurgeon, surgeon, urologist, thoracic surgeon. X-ray, ultrasound of the pleural and abdominal cavity, CT scan, laboratory diagnostics were performed. At the same time, shock management was carried out.Diagnosis: Combined trauma. Open comminuted fracture of the middle third of the right femur with bone fragments displacement. Closed comminuted fractures of the middle third of tibia and fibula with bone fragments displacement. Blunt chest trauma. Bruise of the left lung. TBI, concussion. Sutured wounds of the right thigh and right heel region. Traumatic shock 3rd degree.Initial management: Intensive management of shock, skeletal traction system for both lower limbs and wound surgery, transported to surgical ICU (intensive care unit).
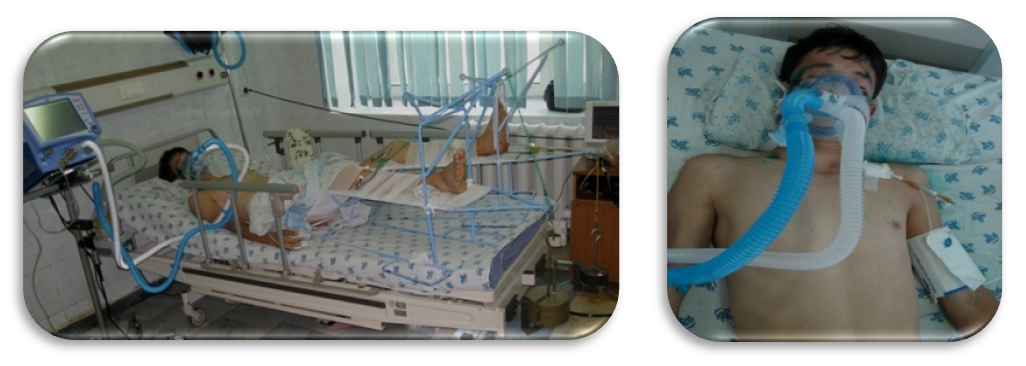 | Figure 2. The patient at 1st day in ICU |
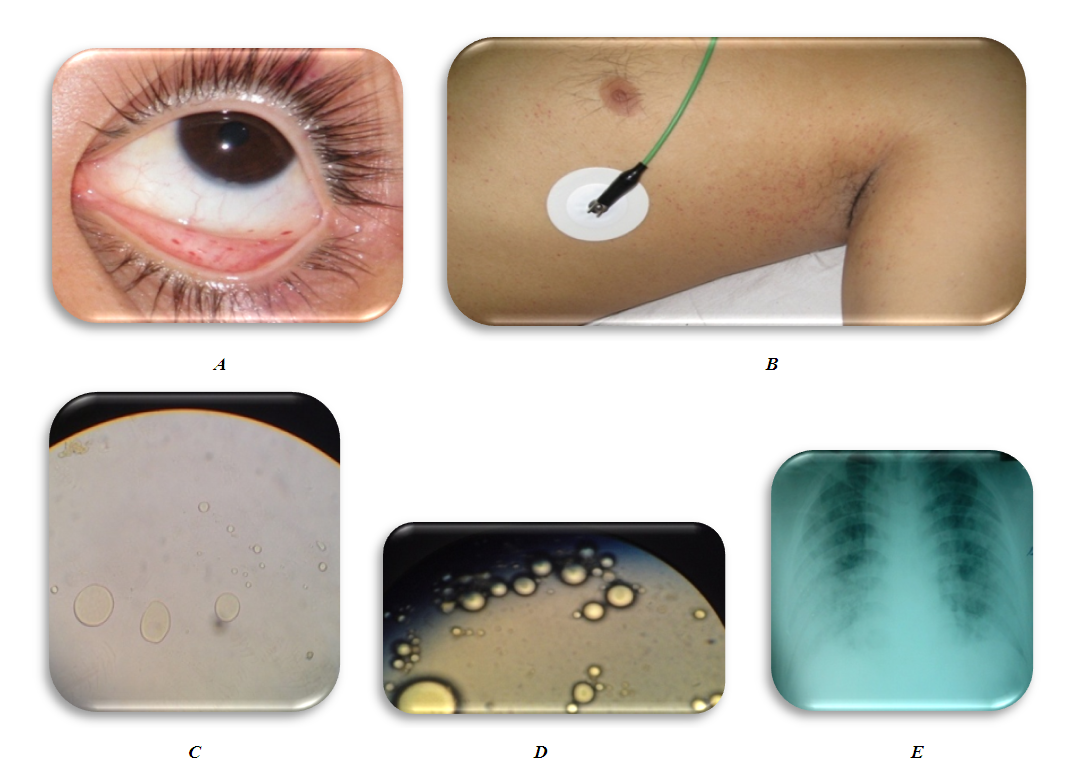 | Figure 3. Initial manifestation of fat globules |
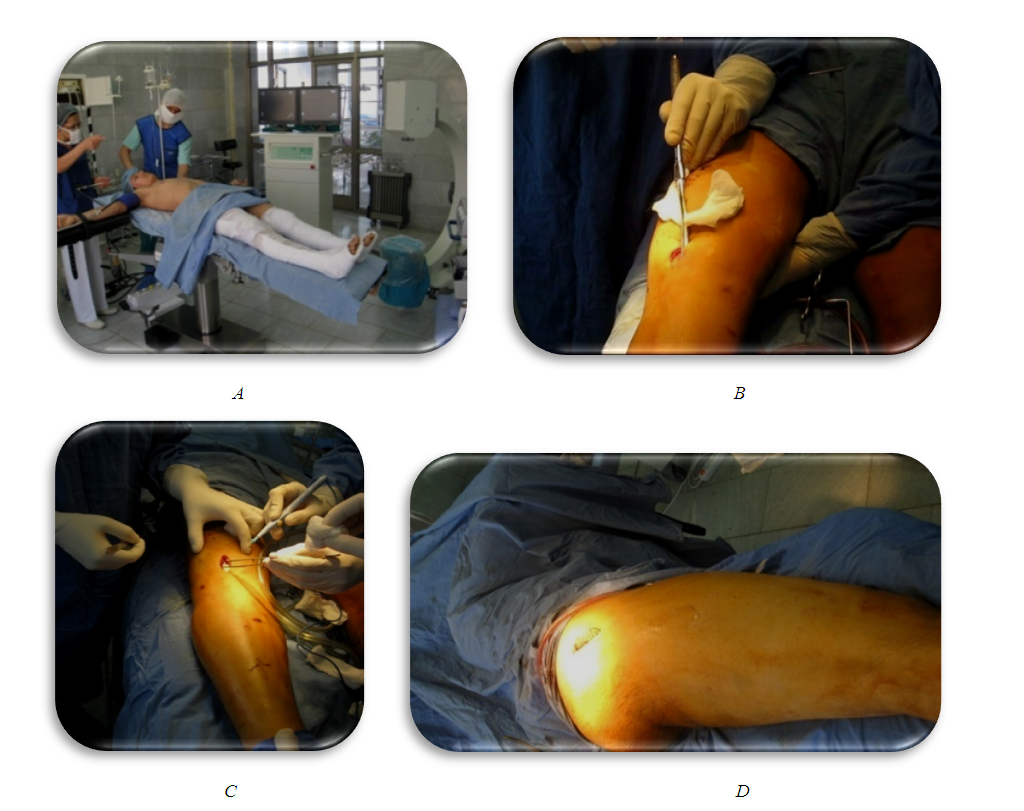
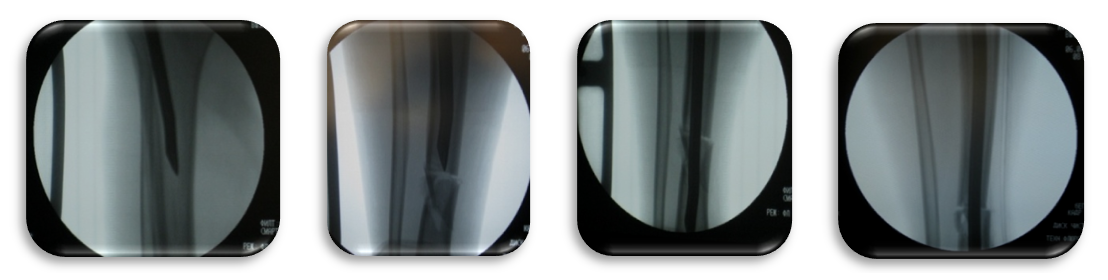 | Figure 5. Intraoperative X-rays of intramedullary osteosynthesis of the left and right lower leg bones under the image intensifier |
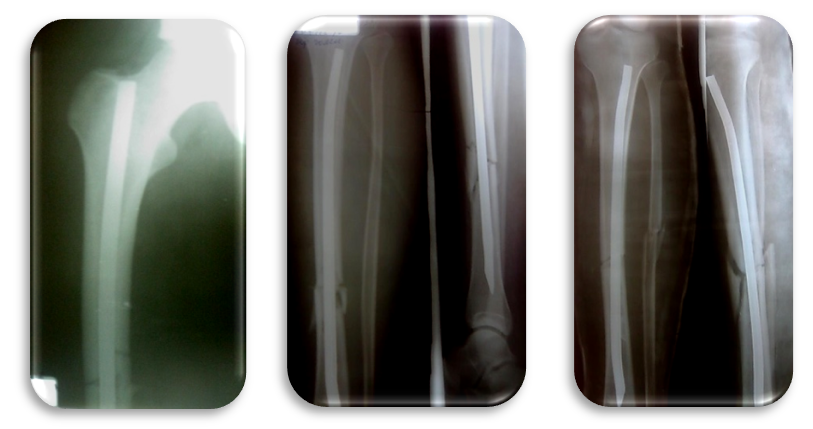 | Figure 6. Immediate postoperative X-rays the right thigh and both tibia after intramedullary osteosynthesis (standing of bone fragments is satisfactory) |
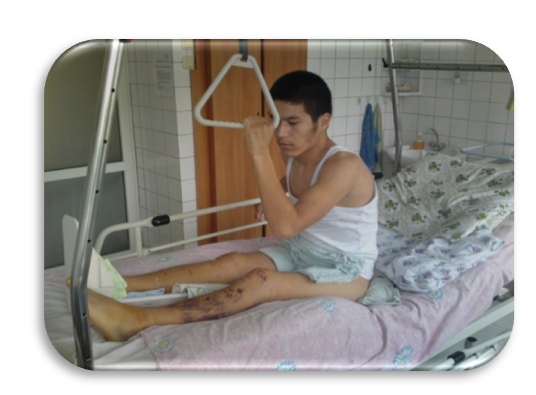 | Figure 7. Patient 8 days after surgery |
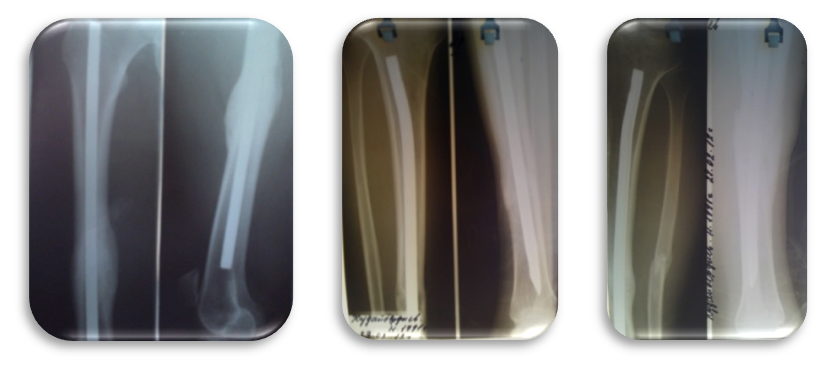 | Figure 8. X-ray 11 months after surgery: complete consolidation of the right and both tibia |
 | Figrue 9. The patient 11 months after surgery |
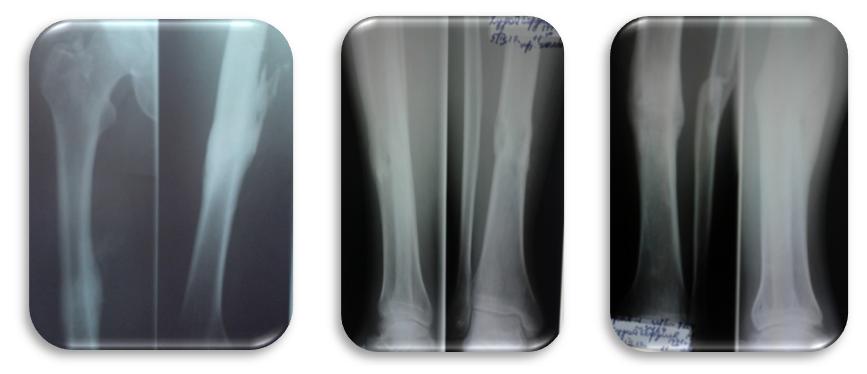 | Figrue 10. X-ray after metal fixators removal (Radiographs of the right thigh and both tibia after metal removal (16 months after surgery)) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML