-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(6): 384-387
doi:10.5923/j.ajmms.20201006.07

Etiopathogenetic Association with Intestinal Parasitosis in Children with Skin Hypopigmentosis
Mirzoyeva M. R., Jabborova O. I., Sagdullaeva G. U.
Bukhara State Medical Institute, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

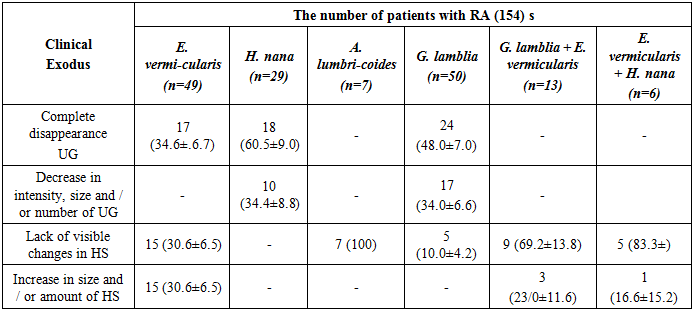
The total IP prevalence in patients with PA in Tashkent (62.0±4.8%) and Bukhara (92.0±2.7%) was higher than in the control gtoups (30.0±4.5%) and (43.0±4.9%) (P <0.05). AT gave the best results in PA patients infected with H. nana and G. lamblia: complete disappearance of hypopigmentation patches (HP) was observed in 60.5% and 48.0% of patients, respectively, and decrease in the intensity, size and/or number of HP was observed in 34.4% and 34.0% respectively. Eventually a positive clinical effect with concomitant hymenolepiasis and giardiasis was observed in 94% and 82% of PA patients. The clinical response was less pronounced in enterobiasis.
Keywords: Hypopigmentoses, Pityriasisalba, E. vermicularis, Microbiology, Antiparasitic
Cite this paper: Mirzoyeva M. R., Jabborova O. I., Sagdullaeva G. U., Etiopathogenetic Association with Intestinal Parasitosis in Children with Skin Hypopigmentosis, American Journal of Medicine and Medical Sciences, Vol. 10 No. 6, 2020, pp. 384-387. doi: 10.5923/j.ajmms.20201006.07.
Article Outline
1. Introduction
- Skin hypopigmentoses include several diseases, in Uzbekistan there are vitiligo and a benign form of hypopigmentosis that affects mainly children and adolescents, and is classified in Western literature as Pityriasisalba (PA). [8]. RAH is characterized by indistinctly defined areas of hypopigmentation (UG), usually oval or rounded, mainly localized on the face (in the main cheeks), arms and upper torso. UG is more noticeable in people with dark skin, and is more common in males [12]. RA does not belong to contagious diseases and so far there are no indications of an infectious etiology of this disease [6,9]. The main complaint of RA patients is a cosmetic defect, but despite this, the patients themselves and their parents usually give an acute negative emotional reaction to the disease. Etiopathogenesis of wounds is Explained, so treatment is usually ineffective and haphazard.Rashiroko is common in countries with tropical and subtropical climates [12,5,7,4,13], usually endemic to helminths and pathogenic protozoa [11]. Uzbekistan is a region endemic to intestinal parasites [1,2], and RA is often diagnosed in the Republic. There is no information about infection with intestinal parasites in RA patients and their possible impact on the development and course of RA. In this regard, the comparative characteristics of RA patients infected with intestinal parasites in two regions of the Republic were of interest.
2. The Purpose
- The purpose of this study was to determine the infestation of intestinal helminths and pathogenic protozoa in RA patients with an assessment of the clinical response to antiparasitic therapy and the possible role of parasites in the pathogenesis of RA.
3. Materials and Methods
- Research was conducted in Tashkent from may 2017 to 2018.d in the research Institute of epidemiology, Microbiology and infectious diseases of the Ministry of Health, in Bukhara - in the same period of time at the Bukhara medical Institute.Groups of patients with CRA in both cities included children aged 5 to 14 years (100 patients in each city). Among RA patients in Tashkent and Bukhara, boys prevailed: 70% and 65%, respectively.The diagnosis of RA was based on a detailed study of the medical history and the results of a clinical examination. Patients were found to have UG with indistinct edges, round or oval shape, size from 0.5 to 5 cm, mainly located on the face, neck, arms, and less often on the upper torso. Some UGS showed mild erythema. The number of UGS varied from 4 to 10. PA diagnosed according to Tenth Revision of International Classification of diseases (ICD-10).Control groups included healthy children (100 people in each city) without any complaints, acute and chronic diseases. In Tashkent, the control group included 78 boys and 22 girls, and in Bukhara — 71 and 29, respectively.All participants in the study are residents of Uzbekistan.Parasitological examination included three-time coproscopy with an interval of 2-3 days. Stool samples were collected in Turdiev's preservative. In case of negative coproscopy results, the modified Ritchiietal concentration method was used. (1952) [10]. Additional examination was performed in RA patients 2 and 6 weeks after completion of antiparasitic therapy prescribed after diagnosis of parasites.Treatment. Antiparasitic therapy was performed after receiving the results of the initial examination and informed consent to treatment of patients and/or their parents.RA patients with ascariasis were treated with a single dose of 400 mg of albendazole.Patients with RAS enterobiosis were prescribed amebendazole (once 100 mg with a repeat after 2 weeks.).RA patients infected with Hymenolepisnana (H. nana) were treated with praziquantel (a single dose of 25 mg/kg with a repeat after 4 days).PA patients infected with Giardialamblia (G. lamblia) were treated with metronidazole (the dose and duration of the course were 15-22.5 mg/kg/day for 10 days).Patients with RA with mixed infection E. vermicularis+G. lamblia were prescribed mebendazole (once 100 mg with a repeat after 2 weeks.) and metronidazole (the dose and duration of the course were 15-22. 5 mg / kg / day for 10 days).RA patients with mixed E. vermicularis +H. nana infection were prescribed mebendazole (once 100 mg with a repeat after 2 weeks.) and praziquantel (a single dose of 25 mg / kg with a repeat after 4 days).Individuals from control groups infected with parasites were also treated using the above-mentioned drugs and regimens. The effectiveness of treatment was evaluated by repeated examination after completing the course of therapy. Patients and their parents were informed about the ways of infecting intestinal parasites and the importance of observing hygiene rules. All RA patients received information sheets on the prevention of intestinal infections.The clinical efficacy of parasite elimination was evaluated 2 and 6 weeks after completion of antiparasitic therapy.Positive clinical effect: complete disappearance and decrease in the intensity of hypopigmentation, the size of UG and their number.The negative clinical effect was characterized by the absence of visible changes in UG or an increase in their number and size.Statistical processing was performed using the Origin 6.1 program (Origin Lab, Northampton, MA). We used methods of variation statistics with the calculation of the arithmetic mean of the studied indicator (M), the mean square deviation (σ), the standard error (m), and relative values (frequency, %). The statistical significance of the obtained measurements when comparing the average values was determined by the student's criterion (t) with the calculation of the error probability (P). The confidence level P<0.05 was taken as statistically significant changes.
4. Results and Discussion
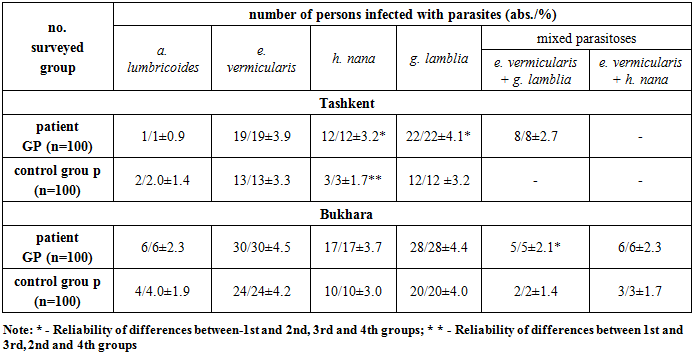
- In table. 1 data for determining intestinal parasite infestation in RA patients and control groups in Tashkent and Bukhara are presented. The total infection rate of RA patients with intestinal parasites in Bukhara was significantly higher than in Tashkent: 92.0± 2.7% and 62.0±4.8%, respectively (P < 0.05). Parasite infestation in the control group was also higher in Bukhara – 43.0± 4.9% and 30.0±4.5%, respectively, but the differences were not reliable (P>0.5). Infection with intestinal parasites in RA patients in Tashkent and Bukharebyl is significantly higher than in the corresponding control groups.
|
|
5. Conclusions
- A positive clinical response to antiparasitic therapy in 60.3% of RA patients infected with parasites indicates the role of helminths and G. lamblia in the development and course of RA and allows us to recommend parasitological examination as the first stage, and in case of detection of parasites, the mandatory appointment of antiparasitic therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML