-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(6): 374-377
doi:10.5923/j.ajmms.20201006.05

Ultrasonic Densitometry and Determination of Calcium Concentration in Blood Serum in Patients with Heavy and Medium Heavy Form Hemophilia
Naimakhon Qobilovna Akhrarova1, Aziza Djumanovna Makhmudova2, Anajon Gazkhanovna Madasheva3
1Hematopoietic Stem Cell Treatment Unit, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Administration, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
3Samarkand State Medical Institute, Samarkand, Uzbekistan
Correspondence to: Naimakhon Qobilovna Akhrarova, Hematopoietic Stem Cell Treatment Unit, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Densitometric parameters and serum calcium concentration were studied in 37 hemophilia patients. Changes in densitometric parameters in patients of this group reliably indicated a decrease in bone mineral density and a decrease in serum calcium. This was the result of frequent fractures in the tubular bones.
Keywords: Hemophilia, Bone mineral density, Diagnosis, Determination of serum calcium concentration
Cite this paper: Naimakhon Qobilovna Akhrarova, Aziza Djumanovna Makhmudova, Anajon Gazkhanovna Madasheva, Ultrasonic Densitometry and Determination of Calcium Concentration in Blood Serum in Patients with Heavy and Medium Heavy Form Hemophilia, American Journal of Medicine and Medical Sciences, Vol. 10 No. 6, 2020, pp. 374-377. doi: 10.5923/j.ajmms.20201006.05.
Article Outline
1. Introduction
- Hemophilia is a rare hereditary disease associated with a violation of the coagulation process. With this disease, hemorrhages occur in the joints, muscles and internal organs, both spontaneous and as a result of trauma or surgery. Patients with severe hemophilia undergo disability due to frequent hemorrhages in the joints (hemarthrosis) and muscle tissue (hematomas). Hemophilia refers to hemorrhagic diathesis due to a violation of the plasma link of hemostasis (coagulopathy).The most severe manifestation of hemophilia is the development of chronic hemophilic arthropathy, the severity of which directly depends on the level of deficient factor and the adequacy of replacement therapy. The clinical course of hemophilic arthropathy begins with acute hemarthrosis, after chronic synovitis, and ultimately leads to deforming osteoarthritis, fibrous ankylosis and the development of secondary periarticular osteoporosis..The combination of hemophilic destructive osteoarthrosis with secondary osteoporosis is the most serious complication for hemophilia patients, which leads to fractures of the tubular bones, most often in metaepiphyseal zones. According to various researchers, the percentage of bone fractures in patients with hemophilia is significantly higher than among a healthy population, and its incidence among this category of patients reaches 15%. In our experience, most often fractures of the tubular bones occur, among which around 68% fall on the femur. Fractures in patients with hemophilia at a young age are associated with an aggressive course of hemophilic arthropathy and a lag in physical development. In this regard, the urgent problem is the timely diagnosis of a local decrease in bone mineral density (BMD) and the conduct of adequate conservative therapy or reconstructive surgery. (Kalis AS, 2012).
2. Main Body
2.1. The Purpose of Our Research
- Of our study is to determine serum Ca and risk factors for osteopenia and osteoporosis in bone metaepiphyses, as well as to trace the dependence of a decrease in bone mineral density in hemophilia patients.
2.2. Material and Methods of Study
- The examination was carried out at the Scientific-Research Institute of Hematology and Blood Transfusion (SRI H&BT) MoH RUz, in the center of hemophilia and depression of hematopoiesis of the city of Tashkent. 37 patients were examined, all of them male: hemophilia A-34 (91.2%) patients and hemophilia B-3 (8.1%) patients. In patient treatment at the age of 18 to 43 years. The average age is 39.5 years. Patients were divided into two groups. The first group (19 people) are patients with severe hemophilia (level of deficient factor VIII / IX <1%), the second group (18 people) are patients with moderate diseases (level of deficient factor VIII / IX from 1 to 5%). An anamnesis was taken, an objective review, anthropometric measurements, general clinical and biochemical examinations were performed. In both groups studied, all patients.The serum calcium concentration was determined by the photometric method. To determine the concentration of calcium in serum, 500 μg of buffer and 500 μg of color reagent and 20 μg of blood serum were used. For determination of blood serum, reagents "HUMAN" (Germany) were used. Measurements were made on a BA88A semi-automatic biochemical analyzer (Mindray, China).And in parallel, ultrasonic densitometry was performed at the Scientific Research Institute of Traumatology and Orthopedics in Tashkent. Measurement of bone density by ultrasound densitometry was performed on an Omnisense 7000S apparatus. Ultrasonic bone densitometer “Sunlight”, (Israel). The method of ultrasonic densitometry is based on standard deviations (BB) embedded in a bone densitometer according to age-related features of changes in bone tissue saturation and mineral density. According to WHO recommendations, BMD assessment is based on the use of standardized T and Z criteria. The T criteria is the result of comparing the bone density of a patient in comparison with a reference value. The norm is 1 point or more. A T-score in the range from –1 to –2.5 is the reason for the diagnosis of “osteopenia” - low mineral density. Less than 2.5 is osteoporosis with a high risk of fractures. The Z-score is the result of comparing the patient’s bone mass density with the average of his age group.Statistical processing of the obtained data was performed using Student's test using the programs "Microsoft Office Excel" and "Biostatistics 4.03". The criterion of statistical reliability was p <0.05.
2.3. Results of the Study
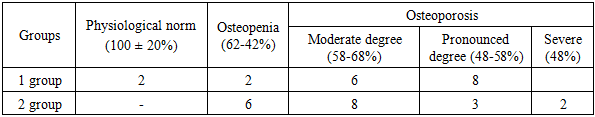
- The results of the study of the concentration of calcium in the blood serum showed that out of 37 patients in 19, the concentration of calcium in the blood was 1.7.mmol / L at the reference values of 2mmol / L, and in 18 patients the concentration of calcium in the blood was 1.8 mmol / L.Of the 37 patients, 25 (67.5 ± 2.03%) patients had bone density of more than 2.5 (BB) T-criteria of 2.5 or less; BMD 68%. The manifestations of osteoporosis. In 10 (27 ± 2.01%) patients with osteopenia (preclinical form of osteoporosis) - bone density is between 1.0 and 2.5 (BB) T-criteria from 1.0 to 2.5 (BB) BMD 68-80%. In 2 (5.4 ± 0.7%) patients, pathology was not detected.Patients with hemophilia were at risk for secondary osteoporosis due to progressive hemophilic arthropathy, a change in movement and physical activity. The accumulation of bone mass and its peak in the puberty in patients with hemophilia depends on active movements, stress on the joints and activity of life. In adults, bone tissue is constantly remodulated: after bone resorption, bone formation occurs. At the cellular level, bone loss occurs as a result of imbalances between the activity of osteoblasts and osteoclasts. The leading role in the process of bone remodulation belongs to the biomechanical load. Physiological metabolism of bone tissue is possible only if there is constant physical activity and active movements in the joints. Low physical activity and the lack of full load leads to a change in bone density, primarily due to a violation of macroarchitectonics (shape and geometry) and microarchitectonics of the bone (ratio of the trabecular and cortical layers). Destructive bone changes in patients with hemophilia are predominantly periarticular in nature and are caused by chronically recurring hemarthrosis, subchondral hemorrhage with the formation of cysts, destruction of articular surfaces, narrowing of the joint space and the development of fibro-bone contractures. Ultimately, this leads to a decrease in the amplitude of movement in the joint, the occurrence of axial deformation of the lower limb and violation of the congruence of the articular surfaces, the destruction of cartilage, muscle atrophy and the occurrence of secondary osteoporosis in the metaepiphysis of the bones that form the joint.The average Z-criterion for any age group is 0.A decrease in Z-criterion to 1.0 or less when examining patients when compared with the average for healthy people of a similar age may indicate the development of osteoporotic processes in the bones. With a loss of bone mass of about 30%, the standard deviation of BMD from the age norm (Z-criterion) is in the range of 2.5-3.0 (BB)..The decrease in BMD and hemophilia are two large and independent pathologies that are interconnected, and their relationship significantly affects the quality of life of the patient with hemophilia. Hemorrhagic manifestations of hemophilia from the musculoskeletal system induce the development of periarticular osteoporosis. Peak bone mass accumulation during puberty is important to prevent the development of secondary osteoporosis and has the greatest protection against the gradual decrease in BMD associated with aging (Mopa and Gilsans, 2003). The full physical load on the bones and joints to form an adequate bone mass in childhood is probably a more important factor than the adequate intake of calcium from food (Bradney, 1998). Patients with hemophilia are at increased risk of developing secondary osteoporosis in childhood, periodic hemarthrosis, joint restriction and the lack of a full load on the bone.A decrease in BMD in hemophilia patients with severe disease (deficiency factor <1%) already at a young age (Barnes et al., 2004) confirms the hypothesis that a young hemophilia patient may never be able to reach peak bone mass compared to healthy contingent (Wallny et al., 2007). Determine serum Ca and risk factors for osteopenia and osteoporosis in bone metaepiphyses, as well as trace the dependence of the decrease in bone mineral density in hemophilia patients.In our studies of the manifestations of osteoporosis in patients with hemophilia, we divided into three degrees of severity:- 1 (moderate) - BMD 56-b6%;- II (expressed) - BMD 46-56%;- III (severe) - BMD less than 46% or less than half of the peak mass.37 patients were divided into two groups. Based on the results of densitometry (Table 1), a significant decrease in BMD in both groups was obtained. For the first group, the median decrease in bone mass in patients with hemophilia was b1.7% (T-test was 3.0 SD), and in the second group - b2.3% (T-test was 2.8 SD). This indicates a significant manifestation of osteoporotic processes in the bones of patients with hemophilia compared with the age of the bone mass of a healthy population. Almost the same percentage decrease in BMD in both groups and the coincidence of the T-criteria may indicate insignificant significance. The manifestations of the disease, associated primarily with hemophilic arthropathy, come to the fore in the development of osteoporosis in hemophilia patients.
|
3. Conclusions
- The obtained decrease in BMD in patients with hemophilia with osteoarthritis in adulthood characterizes a pronounced violation of the processes of osteogenesis in the periarticular zone.Early detection of osteopenia in children with hemophilia with hemophilic arthropathy can prevent the development of secondary osteoporosis in adulthood.Early detection of osteopenia in children with hemophilia with hemophilic arthropathy can prevent the development of secondary osteoporosis in adulthood.Densitometric examination indices can be recognized as a diagnostic criterion for the possibility of the occurrence of "pathological" fractures of the tubular bones in patients with hemophilia with damage to the musculoskeletal system.
ACKNOWLEDGEMENTS
- We express our gratitude for the help in the clinical and instrumental examination of patients to employees of the Scientific Research Institute of Traumatology and Orthopedics in Tashkent.We also want to express our gratitude to the staff of the clinical diagnostic department of the Scientific-Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan for their help in organizing laboratory biochemical blood tests of the examined patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML