Mirzakhmedova Dilfuza1, Salikhova Kamola2, Ishniyazova Nadira3
1Junior Researcher, Republican Specialized Scientific and Practical Medical Center of Pediatrics, Tashkent, Uzbekistan
2Head of the Department of Neonatology, MD, Republican Specialized Scientific and Practical Medical Center of Pediatrics, Tashkent, Uzbekistan
3Senior Researcher, Republican Specialized Scientific and Practical Medical Center of Pediatrics, Associate Professor, Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Correspondence to: Mirzakhmedova Dilfuza, Junior Researcher, Republican Specialized Scientific and Practical Medical Center of Pediatrics, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of studies of 95 preterm infants with respiratory distress syndrome and bronchopulmonary dysplasia, hospitalized in the ICU and ARF of the Republican Specialized Scientific Practical Medical Center of Pediatrics in Tashkent. Analysis of the results of the study showed that in children with bronchopulmonary dysplasia there is an increase in the level of pro-inflammatory cytokines, such as IL 1β, IL 6, TNF α. In a study on the development of bronchopulmonary dysplasia after suffering respiratory distress syndrome, theVascular endothelial growth factor (VEGF) was lower compared to children who did not develop BPD.Thus, the obtained data showed that a complex determination of the level of cytokines and VEGF in the serum of newborns with respiratory disorders can be used in clinical neonatology as informative for the diagnosis of severity and prognosis of its adverse outcomes due to immaturity of newborns.
Keywords:
Prematurity, Respiratory distress syndrome, Bronchopulmonary dysplasia, Newborns
Cite this paper: Mirzakhmedova Dilfuza, Salikhova Kamola, Ishniyazova Nadira, The Role of Vascular Endothelial Growth Factor (VEGF) and Pro-Inflammatory Cytokines in the Pathogenesis of Bronchopulmonary Dysplasia in Preterm Infants, American Journal of Medicine and Medical Sciences, Vol. 10 No. 5, 2020, pp. 333-338. doi: 10.5923/j.ajmms.20201005.12.
1. Introduction
One of the etiological factors for the high morbidity of children born prematurely is the immaturity of the respiratory and immune systems, which causes the greatest susceptibility of deeply premature babies to the development of such a pathological condition as bronchopulmonary dysplasia (BPD). According to the modern definition, bronchopulmonary dysplasia is a polyetiological, multifactorial chronic disease of morphologically immature lungs, developing in newborns, mainly deeply premature, as a result of intensive care of respiratory distress syndrome (RDS) and pneumonia.BPD occurs bythe lesions of the bronchioles and lung parenchyma, the development of emphysema, fibrosisor impaired alveolar replication [1,2,4,7]. Despite significant progress in understanding the mechanisms of development, diagnosis, therapy and prevention of BPD, there are a number of scientifically-practical problems of this pathology [10,11,12,21]. In the development of BPD, a significant place is given to the “vascular” hypothesis, according to which this disease reflects a disorder of the evolution of pulmonary angiogenesis with subsequent impaired development of the alveoli [15,23].Lung angiogenesis is largely regulated by VEGF, which is the main mediator of vascular permeability, proliferation and migration of endothelial cells, important for vasculogenesis and angiogenesis.In lung development, VEGF regulates vascularization, but its content varies throughout this process. In the embryonic lung, VEGF is found in primitive airway epithelium and mesenchyme, but at the pseudoglandular stage, VEGF is expressed mainly by branching airway epithelial cells. In mature lungs, VEGF is expressed by type II epithelial cells [8,19,23].The level of VEGF expression that is synthesized in the endothelium of the microvascular vessels of the respiratory tract is crucial in the induction of angiogenesis, the development of the lungs, and the maintenance of their structures. As it is known, VEGF promotes the secretion of surfactant and lung maturation. In this connection, the importance of VEGF in the development of BPD has been noted in many studies [13,15,17,23].Also, in the development of BPD in newborns, immunological risk factors are of no small importance.Imbalance of pro and anti-inflammatory cytokines in children of different gestational age with respiratory disorders is an urgent task for the diagnosis, prognosis, course of diseases and the choice of treatment tactics [11,17,22].As known, one of the main predictors of mothers of premature babies is chorioamnionitis. There is a connection between chorioamnionitis and BPD of premature newborns, which is explained by the development of the autoimmune process. As a result of the immune response, interleukins (IL)-1.6 and tumor necrosis growth factor (TNF-α), are produced. In addition, in response to mechanical ventilation in newborns with high tidal volumes, the activation of these pro-inflammatory markers is observed. As a result of exposure to these interleukins, alveolarization and vascularization are disrupted, and these factors themselves can be considered predictors of BPD [2,5,6,14].All this indicates the need to search for early biomarkers of BPD predictors in newborns.
2. The Purpose of the Study
Study of the role of vascular endothelial growth factor and pro-inflammatory cytokines in the development of bronchopulmonary dysplasia in premature infants.
3. Materials and Methods
We examined 95 newborns who were treated in the neonatal department of the RSNPMC Pediatrics and the Republican Perinatal Center. Children were divided into 2 groups. The 1st group consisted of 38 children with a diagnosis of BPD. The 2nd group included 57 children who did not have BPD. Of the children examined, 60 newborns had a gestational period of 31.3 ± 0.2 weeks, very low body weight (VLBW) of 1608.5 ± 125.47 grams, and a body length of 42.9 ± 1.2 cm; 35 newborns with a gestation period of 28.8 ± 0.12 weeks, an extremely low body weight (ELBW ) of 855.5 ± 75.8 g, a body length of 37.3 ± 2.5 cm.Exclusion criteria were newborn children with congenital heart defects, hereditary, chromosomal diseases.The diagnosis of BPD was established according to modern criteria: damage to the bronchioles and lung parenchyma, development of emphysema, fibrosis and impaired replication of the alveoli, oxygen dependence at the age of ≥ 28 days of life, bronchial obstruction syndrome and symptoms of respiratory failure, characteristic data of an x-ray picture of the lungs. The degree of respiratory failure at birth was evaluated on a Silverman scale.Intensive care was a standard program of etiological and syndromic therapy.The amount of vascular endothelial growth factor (VEGF) and the cytokine spectrum of interleukins IL 1, TNF a, IL 6 were determined by the biochip method using the Randox apparatus.Static processing of the results was carried out using the Microsoft Excel Version 7.0 by application of mathematical-statistical analysis. The significance of differences in statistical populations was evaluated by parametric methods for various variances using Student's criterion. To identify the relationship between the studied parameters, Pearson's linear correlation analysis was used.
4. Results and Discussions
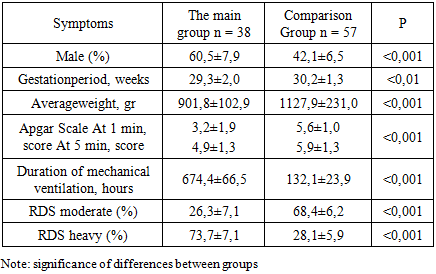
The clinical characteristics of the children in the examined groups were carried out in dynamics and included a detailed study of somatic status, adequate to the severity of the child's condition. A comparative assessment of the general clinical signs of newborns with BPD and without BPD is presented in table 1. At the same time, special attention was paid to indicators of monitoring the vital functions of the body of newborns.Table 1. Comparative general clinical characteristics of newborns
 |
| |
|
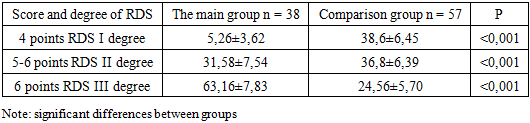
Assessment of the condition of children in the early neonatal period showed that, the vast majority of those examined were born in serious condition. The severity of the condition was due to respiratory failure, deep immaturity suffered by chronic intrauterine and acute hypoxia during childbirth. All children were born in a state of moderate to severe asphyxiation. The condition of newborns, who needed an extended mechanical ventilation was regarded as very serious.Assessment of the severity of RDS using the adapted Silverman scale are presented in table 2. At the same time, respiratory failure of the II and III degree was more often observed in newborns of the main group 31.5% and 63.15 respectively, while in children of the comparison group there was more often respiratory failure of the I and II degrees of 38.6% and 36.8% of cases, respectively.Table 2. The degree of RDS in newborns of the examined groups on the Silverman scale (%)
 |
| |
|
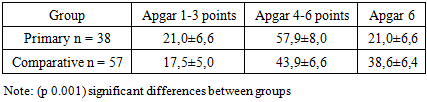
As can be seen from the table, in all groups of newborns there is a clinic of respiratory failure.In order to stop the symptoms of respiratory failure, all newborns underwent respiratory therapy. Newborns with RDS 1 to 2 degrees more often required non-invasive mechanical ventilation using the CPAP method using binasal cannulas with an oxygen concentration of 30–50% of the inhaled air mixture or oxygen supply through an oxygen mask. By the end of the neonatal period, 38 newborns developed BPD. The diagnosis of BPD was carried out on the basis of anamnesis, the presence of need for additional oxygen at the age of 28 days of life, physical examination, symptoms of respiratory failure (tachypus, wheezing during auscultation, chest retraction during breathing and the need for oxygen support) and characteristic changes in chest radiographs. On the chest x-ray, signs of BPD were detected in the form of interstitial fibrosis, enrichment of the pulmonary pattern due to the vascular component.Children with RDS were also given comprehensive prophylaxis of BPD with systemic and inhaled steroids according to the standard scheme. All newborns with BPD cases were in the intensive care unit on mechanical ventilation for respiratory distress syndrome. The frequency of BPD development decreased with increasing body weight and gestational age: for example, in the newborns examined by us, BPD developed in 8 children with very low birth weight (VLBW), which corresponded to 21.1% and 30 born with) extremely low birth weight (ELBW), which corresponded to 78.8% of cases. Significant differences in the sexual composition of newborns were obtained, indicating a male predisposition to the development of BPD.First of all, there is a significant difference in anthropometric indicators at birth and gestational age (p 0.001). A severe form of BPD was developed by newborns born earlier than 29 weeks of gestation, weighing less than 1000 grams, who had a lower Apgar score at birth. In children of both groups, the presence of asphyxia of the newborn in the first minutes of life was noted (Apgar score below 5-6 points), which is essential for determining the prognosis of adverse outcomes of RDS in preterm infants. According to the data obtained, the estimate at the 1st minute of life was significantly lower in all groups of newborns that formed BPD.Correlation analysis showed a direct statistical relationship between birth weight and Apgar scores at 1st minute (r = 0.70; p 0.001). In children with ELBW and BPD, the rating for this scale at the 5th minute of life was also significantly lower compared to children without BPD. Newborns with an Apgar score of less than 5 points in the fifth minute needed primary resuscitation and transfer to mechanical ventilation in connection with severe respiratory failure. The criteria for the transfer to mechanical ventilation were: violations of the nature and rhythm of breathing (tachypnea, periodically occurring attacks of bradypnea, up to apnea); changes in the acid-base balance of the blood (decrease in the partial pressure of oxygen, hypercapnia, decrease in pH). The average duration of artificial ventilation of newborns of the main group and the comparison group averaged 674.4 ± 66.5 and 132.1 ± 23.9 hours respectively (p 0.001). Of the examined newborns with RDS, 22 (23.2%) developed pneumonia, of which 12 were newborns with ELBW and 10 with VLBW.Table 3. Apgar score at birth in newborns (%)
 |
| |
|
Thus, severe RDS, long-term need for mechanical ventilation and the development of pneumonia were associated with the formation of BPD in newborns with ELBW and VLBW. An analysis of perinatal factors revealed that a gestational age of less than 29 weeks was the leading risk factor for BPD. An patent arterial duct (PDA) is also a risk factor for BPD, which is observed in 55–66% of newborns weighing less than 1500 g.Left-right discharge in case of PDA is the cause of excessive blood supply to the lungs and an increase in pressure in the pulmonary artery.The increased permeability of the pulmonary capillaries of premature infants and low oncotic plasma pressure lead to the sweating of fluid into the lumen of the alveoli and inactivation of surfactant [16,17,18].The next stage of our study was aimed at studying the possibility of predicting the course and outcome of the disease based on a comparative analysis of the studied parameters of both groups.In newborns with symptoms of respiratory failure, a study of inflammation indicators was conducted: IL-1β, IL-6 and TNF-α on the 7th and 14th days of life to determine the pathogenetic mechanism of the development of RDS and its complications.The course of the systemic inflammatory response in patients of the main groups is characterized by an increase in the level of all types of cytokines by the 7th day of life, i.e. the course of the systemic inflammatory response in them is determined by the activity of pro-inflammatory cytokines.As can be seen from the data presented (table 4), the content of all pro-inflammatory cytokines in children who formed BPD on days 7-14 was statistically significantly increased compared with newborns without BPD (p<0.001).Table 4. The level of serum cytokines in newborns of different gestational age on the 7th and 14 th day of life
 |
| |
|
As a result of the immune response, the production of pro-inflammatory cytokines is enhanced. At a high concentration, the TNF-α can damage endothelial cells and increase microvascular permeability. This causes activation of the hemostatic system and complement, followed by the accumulation of neutrophils and microvascular thrombosis. As a result of the influence of these factors, alveolarization and vascularization are disordered, and these factors themselves can be considered predictors of BPD [7,12,15].We studied the correlation between the concentrations of pro-inflammatory cytokines in blood serum and such factors as gestational age, body weight at birth, Apgar score at 1st and 5th minutes of life, and duration of mechanical ventilation. At the same time, a significant dependence was revealed in children, i.e. negative correlation between TNF-α level and Apgar score at the 5th minute of life (r=-0,65, р<0,05). This fact suggests that severe hypoxia leads to the development of critical conditions in the early neonatal period and is accompanied by severe inhibition of the immunological reactivity of the fetus and newborn. Analysis of the cytokine content depending on gestational age confirms that the concentration of pro-inflammatory cytokines is inversely correlated with gestational age and body weight at birth. It was revealed that hyperactivation of the cytokine system correlates with a disorders of thrombotic resistance of the vascular endothelium. This is probably due to the procoagulant activity of IL-1β, IL-4, TNF-α, which is determined by the ability of these cytokines to enhance the expression of tissue factors, reduce the expression of thrombomodulin, and stimulate the synthesis of tissue plasminogen activator, which against the background of vasoconstriction of arterioles leads to disruption of microcirculation, the development of hypoxia and lack of energy supply to tissues, necrotic and apoptotic processes in the site of inflammation. The persisting high concentration of pro-inflammatory cytokines is an unfavorable factor reflecting the activity and severity of the pathological process [15,20,22].In the development of BPD in newborns, a significant place is given to the “vascular” hypothesis, according to which this disease reflects a disorder of the process of pulmonary angiogenesis with subsequent impaired development of the alveoli. Angiogenesis is closely associated with inflammation, as the production of pro-angiogenic factor is regulated by such cytokines as VEGF, IL-1β, TNF-α. VEGF may promote surfactant secretion and lung maturation. [3,9,22]. In order to determine the relationship between the maturity and severity of the condition of the newborn and the level of VEGF, we studied the content of this cytokine in the newborns examined by us. The content of VEGF in serum began to be determined in newborns from 6 to 10 days of life.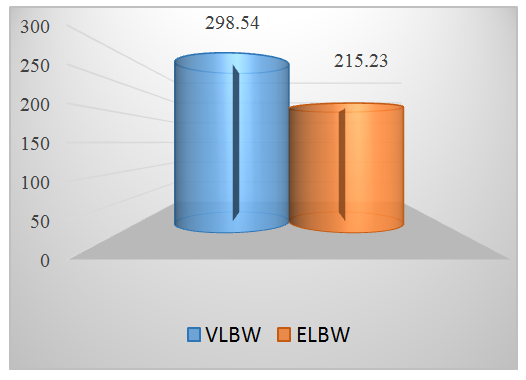 | Figure 1. The level of VEGF in newborns depending on body weight and gestational age (pg / ml) (p 0.001) |
The VEGF level in newborns with VLBW was 298.54 ± 53.9 pg / ml. In newborns with ELBW, this indicator was 215.23 ± 34.0 pg / ml. When studying the role of VEGF in the development of an adverse outcome of respiratory failure in premature infants, we found a significant difference in the performance of newborns with BPD and without BPD.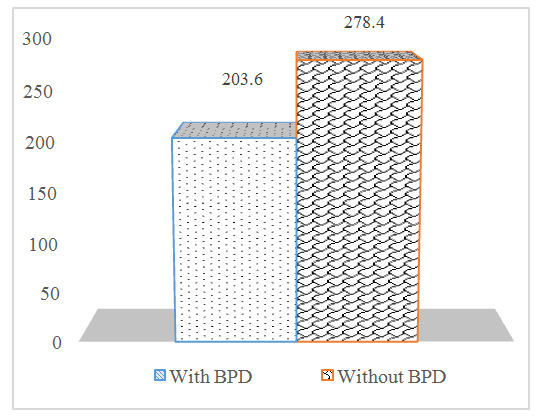 | Figure 2. Serum VEGF levels in infants with and without BPD (pg / ml) |
From the presented results, it follows that VEGF may contribute to the secretion of surfactant and lung maturation. In a study on the development of bronchopulmonary dysplasia after undergoing RDS, premature babies with more severe RDS had a low level of VEGF compared to children in whom it did not develop.Low VEGF concentrations in premature babies with more severe RDS may contribute to the development of acute lung damage. In children with bronchopulmonary dysplasia, a significant decrease in VEGF was observed (р<0,05), indicating inhibition of angiogenesis, activation of fibrosis and endothelium of dependent vasodilation factors from the first months of life. Higher blood VEGF values were found in premature infants with mild forms of RDS.In the study of blood serum in preterm infants with RDS, it was found that a large prematurity period and low weight, longer mechanical ventilation with tighter parameters, as well as low Apgar values, correlate with lower blood VEGF values. Considering the literature data on the tendency for vasoconstriction to predominate in premature babies, we can conclude that the slowing of angiogenesis reduced the volume of the pulmonary vascular network, increased pressure in the pulmonary vessels and contributed to the disruption of matrix synthesis. Increased pressure in the pulmonary artery, in turn inhibited the growth of pulmonary vessels.
5. Conclusions
The results of our studies showed that a comprehensive determination of the level of pro-inflammatory cytokines in serum in newborns with respiratory disorders can be used in clinical neonatology as an informative for the diagnosis of severity and prognosis of adverse outcomes due to immaturity of the newborn. A significant decrease in the content of vascular endothelial growth factor involved in the processes of growth and remodeling of endothelial cells and vascularization of the lungs leads to impaired alveolarization and angiogenesis, the formation of a pathoanatomical picture, similar to BPD [1,15,23]. Reducing the level of VEGF in serum can be used for early prediction of the development of BPD in this contingent of children.
References
| [1] | Belyaeva I.A., Davydova I.V. The role of genetic factors in the formation of bronchopulmonary dysplasia in children // Voprosi diagnostiki v pediatrii. - 2012. - T. 4. - No. 5 - S. 5–9. |
| [2] | Davydova I.V., Anikin A.V., Kustova O.V., et al. Bronchopulmonary dysplasia in the post-surfactant era: results of an objective assessment of the course of the disease // Voprosi sovremennoy pediatrii. - 2015. - T. 14. - No. 4 - S. 514–518. |
| [3] | Frank, L. Antioksidans, nutrition, and bronchopulmonary dysplasia / L. Frank // Clinic in perinatal. - 1992. - Vol. l9. - No. 3. - R. 541-561. |
| [4] | Genetics of bronchopulmonary diseases / Ed. V.P. Puzyreva, L.M. Gardening. - M.: Atmosfera, 2010.-- 160 p. |
| [5] | Heytmanek G., Salzer H., Vityska- Binstorfer E., Genger H., Metka M., Pfersmann R., Wolff F., Weidinger H. Ambroxol versus betamethasone for the promotion of antepartum lung maturity in pathological pregnancies. Wien Klin Wochenschr., 1990 Aug 3. № 102 (15). Р. 443—448. |
| [6] | Howard, J. Reduction in the incidence of chronic lung disease in very low birth weight infants: results of a quality improvement process in a tertiary level neonatal intensive care unit / J. Howard // Pediatrics. - January. - 2009. -- 123 (1). - R. 44 - 50. |
| [7] | Ionov O.V., Degtyarev D.N., Prutkin M.E. et al. Management of newborns with respiratory distress syndrome. Guidelines edited by academician of the Russian Academy of Medical Sciences N.N. Volodina // Neonatologiya. - 2014. - No. 1 (3). - S. 129-144. |
| [8] | Maniscalco WM, Bhandari V. Disruption of lung microvascular development. In: Abman SH, editor. Bronchopulmonary Dysplasia. Informa Healthcare; New York: 2010. pp. 146–166. [GoogleScholar] |
| [9] | Modern approaches to the prevention, diagnosis and treatment of bronchopulmonary dysplasia: a guide for practitioners. / Ed. A.A. Baranova, L.S. Namazova-Baranova, I.V. Davydova. - M.: Pediatr, 2013. -- 176 p. |
| [10] | Ovsyannikov D. Yu. Bronchopulmonary dysplasia: questions of terminology and classification / D. Yu. Ovsyannikov, I.V. Davydova // Rossiyskiy pediatricheskiy jurnal. - 2008. - No. 2. - S. 18-23. |
| [11] | Ovsyannikov D.Yu., Antonov A.G., Ionov O.V. et al. Draft protocol for the diagnosis, prevention and treatment of bronchopulmonary dysplasia // Neonatologiya. - 2014. - No. 1. - S. 161-175. |
| [12] | Panov P.V. Perinatal and immunogenetic risk factors for bronchopulmonary dysplasia Avtoref. dis. kand. med.nauk.:. - M.; 2015.-- 22 p. |
| [13] | Panov P.V., Akhmadeeva E.N., Panova L.D., Baykov D.E. Perinatal history and genetic aspects of the formation of bronchopulmonary dysplasia in deeply premature infants // Prakticheskaya medicina - 2013. - No. 7 (76). - S. 131-135. |
| [14] | Panov P.V., Panova L.D., Yarukova E.V., Akhmadeeva E.N. Prognostic risk factors for the formation of bronchopulmonary dysplasia in premature infants M: Prakticheskaya medicina 2016 №3 p46-53. |
| [15] | Panchenko A.S. Pathogenetic characteristic and prediction of the formation of bronchopulmonary dysplasia in premature infants: Avtoref. dis. … dokt. med.nauk.. - Irkutsk; 2015.-- 43 p. |
| [16] | Pavlinova E.B., Krivtsova L.A., Sinevich O.Yu. Prediction of the risk of developing bronchopulmonary dysplasia in premature infants // Pediatriya - 2012. - T. 91, No. 2. - S. 23-29. |
| [17] | Pavlinova E.B. Analysis of polymorphism of the antioxidant system enzyme genes in preterm infants at risk for the formation of bronchopulmonary dysplasia // Voprosi diagnostiki v pediatrii - 2011. - T. 3. - No. 5 - S. 14–19. |
| [18] | Ramet, M. Association between the surfactant protein A (SP-A) gene locus and respiratory-distress syndrome in the Finnish population / M. Ramet, R. Haataja, R. Marttila // Am. J. Hum. Genet. - 2000. - May. - 66 (5). - R. 1569 - 79. |
| [19] | Randomized, Controlled Trial of Dexamethasone in Neonatal Chronic Lung Disease: 13-to 17-Year Follow-up Study: I. Neurologic, Psychological, and Educational Outcomes / A. K. Rosamond [et al.] // Pediatrics. - August. - 2005. -- R. 116: 370 - 378. |
| [20] | Rivera L, Siddaiah R, Oji-Mmuo Ch, et al. Biomarkers for Bronchopulmonary Dysplasia in the Preterm Infant. Frontiers in Pediatrics. 2016; 4: 33. Availableat: https: //www.researchgate.net / publication / 299554677 (published online 03/31/2016. |
| [21] | The scientific and practical program "Bronchopulmonary dysplasia." - M.: Original layout, 2012. -- 88 p. |
| [22] | The baud B., Lacaze Masmonteil T. If your placenta doesn’t haveit either: The “Vascular Hypothesis” of bronchopulmonary dysplasia starts in utero // J. Pediatr. - 2010. ― No. 156. - R. 521-523. |
| [23] | Zakharova N.B., Durnov D.A., Mikhailov V.Yu. and other Diagnostic value of the study of vascular endothelial growth factor in blood serum // Fundamentalnye issledovaniya. - 2011. -№11. - S. 215-220. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


