-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(5): 293-297
doi:10.5923/j.ajmms.20201005.04

Comparative Assessment of the Immediate Results after Exenteration of Pelvic Organs with Simultaneous Pelvic Floor Reconstruction in Patients with Local Extended Cervical Cancer
Zakhirova N. N., Tillyashaykhov M. N., Adylkhodjaev A. A., Akhmedov O. M., Umarova N. A., Osmanova E. Z., Saidakhmedova V. A.
Republican Specialized Scientific and Practical Center of Oncology and Radiology, Tashkent, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study analyzed the treatment results of 141 patients with locally extended cervical cancer with T2b-4N0-1M0 stage of disease after anterior pelvic exenteration. The study compares the immediate and long-term results after anterior pelvic exenteration with and without performing the reconstructive phase of the surgery. The age of patients ranged from 32 to 61 years, the mean age was 47.1 years. The authors made a comparative assessment of postoperative complications in 62 (44%) patients after pelvic exenteration without plastic reconstruction of the pelvic floor and in 79 (56%) patients who underwent the reconstructive stage. The possibilities and advantages of pelvic floor plastic reconstruction which reduce the incidence of postoperative complications have been evaluated.
Keywords: Exenteration, Pelvis, Cancer, Cervix, Plastic reconstruction
Cite this paper: Zakhirova N. N., Tillyashaykhov M. N., Adylkhodjaev A. A., Akhmedov O. M., Umarova N. A., Osmanova E. Z., Saidakhmedova V. A., Comparative Assessment of the Immediate Results after Exenteration of Pelvic Organs with Simultaneous Pelvic Floor Reconstruction in Patients with Local Extended Cervical Cancer, American Journal of Medicine and Medical Sciences, Vol. 10 No. 5, 2020, pp. 293-297. doi: 10.5923/j.ajmms.20201005.04.
1. Introduction
- Cervical cancer (CC) takes the first place among the pathologies of the genitals in developing countries and the third one after cancer of the body of the uterus and ovaries in economically developed countries [1]. According to the WHO, about 370000-500000 new cases of the disease are detected every year in the world. Moreover, in approximately 50% of cases, the disease is already detected at locally extended stages, therefore, the five-year survival rates after surgical treatment of locally advanced cancer of the pelvic organs do not exceed 27.2%. Treatment of patients with locally extended primary and recurrent forms of cervical cancer remains a largely unsolved problem [2-4].In patients with locally extended forms of cervical cancer (LECC) (when a cervical tumor invades the bladder or rectum), exenteration of small pelvis (ESP) organs is performed: anterior, posterior, or total, depending on the extent of the tumor process. Pelvic exenteration is characterized by high technical complexity and laboriousness, in terms of its effect on the patient’s body it is a very traumatic surgery and belongs to the category of “super-aggressive” surgical interventions [5-6].When removing the pelvic organs as a single unit, an extensive pelvic floor defect is formed. After ESP, translocation of the small intestine loops into the pelvic cavity, their fixation by adhesions to the pelvic walls and the pelvic floor devoid of peritoneal cover occur; the consequences of radiation therapy are evident pelvic fibrosis, vascular hyalinosis, worsening of vascularization of the pelvic tissues [7-9]. Fixation of the small intestine loops to the irradiated walls of the pelvis can lead to the formation of intestinal obstruction, small intestinal fistulas, recto-vaginal fistulas, lymphocele and abscesses of the small pelvis and pelvic hernias. The above-described group of complications is called "syndrome of empty small pelvis "in the literature [10-12]. A natural question how to fill the empty space in order to prevent the translocation of the small intestine loops into the pelvic cavity arises. Currently, various reconstruction methods are used: omento-plasty, plasty with muscle and skin-muscle flaps, transposition of the blind and sigmoid colon as well as the use of absorbable and non-absorbable materials such as mesh implants [13-15]. Aim: to study the treatment results of patients with locally extended cervical cancer after anterior exenteration of small pelvis organs depending on various methods of pelvic floor reconstruction.
2. Material and Methods
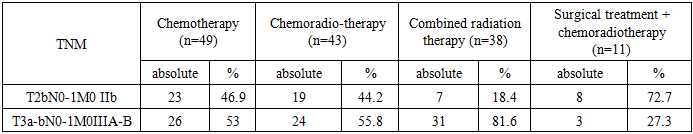
- We studied 141 patients with locally extended forms of cervical cancer (LECC) with invasive growth into the bladder, T2b-4aN0-1M0 stage of the disease, with a complicated course, during chemoradiotherapy or relapse of the disease after combined or complex treatment. 12 of the total number of patients were initially identified patients with stage T4aN0-1M0. All studied patients were divided into 2 groups, control and main, and the main group was divided into 3 subgroups (Fig 1).
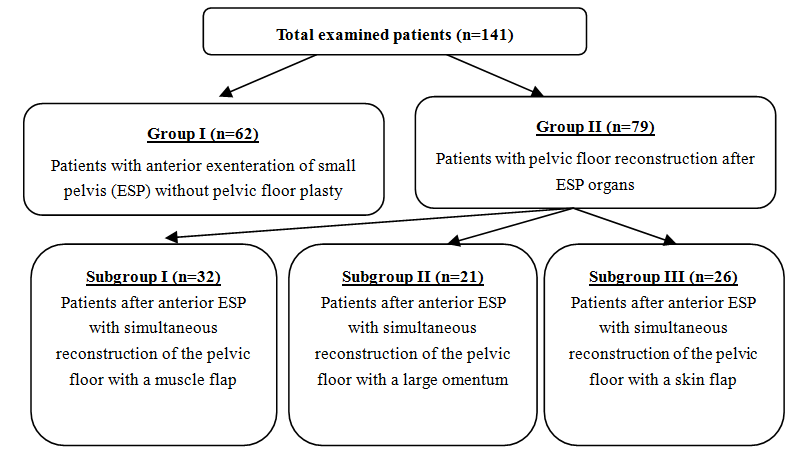 | Figure 1. The distribution of patients in the studied groups |
 | Figure 2. The distribution of patients by age |
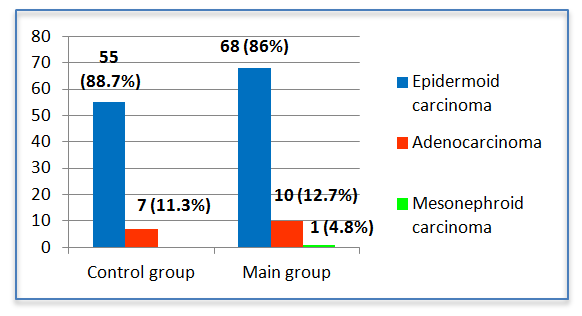 | Figure 3. The distribution of patients according to the morphological structure of the tumor |
|
3. Results and Discussion
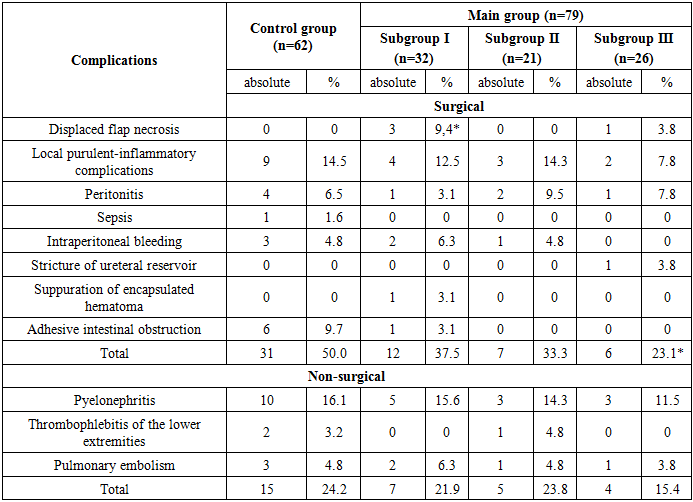
- All studied patients were performed anterior ESP with a simultaneous reconstructive stage. The reconstructive stage of the operation at the anterior ESP implies:1) The choice of urine diversion method; 2) Pelvic floor restoration. This stage of the operation significantly affects the problems of patients’ postoperative rehabilitation and also determines the quality life of their life.In our study we applied 3 methods of urine diversion: ureterocutaneostomy (UCS) (n = 81) - 57.4%; Bricker’s operation (n = 34) - 24.1% and a heterotopic urinary bladder with appendicostomy (n = 26) - 18.4%. Another objective of the ESP reconstructive phase is the reconstruction of the pelvic floor. In patients of the subgroup I (n = 32), for the reconstruction of the pelvic floor we used the external and internal obturator muscles, the levator muscle of the rectum and the piriformis muscle which were sewn together with vicril in the form of a cascade. In patients of subgroup II (n = 21), a large omentum from which a J-shaped flap was formed on the right or left gastrointestinal vessels was used to reconstruct the pelvic floor, after mobilization from the transverse colon and large curvature of the stomach, it was reduced to the pelvic cavity and was fixed with separate vicryl sutures to the walls of the pelvis and the pelvic floor. For the third subgroup of patients (n = 21) a skin flap which was excised with a subcutaneous fat inlet from the anterior abdominal wall measuring 6x7x12 cm was used for pelvic floor reconstruction. After that, the epidermis layer of the skin flap was removed to the layer of its own dermis, then the formed skin flap was installed in the area of the “empty” pelvic floor and fixed to the pelvic peritoneum.Assessment of treatment results in patients was carried out in the early (up to 5 days after surgery), late (up to 30 days after surgery) and the long-term postoperative periods (30 days or more after surgery). Table 2 shows the results of a comparative assessment of the nearest complications which are divided into surgical and non-surgical.
|
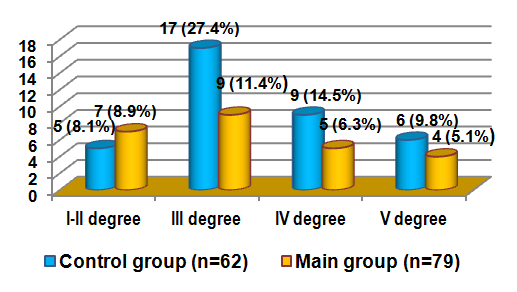 | Figure 4. Distribution of postoperative complications by the Clavien-Dindo scale |
|
4. Conclusions
- Thus, we can conclude that in patients with locally extended forms of cervical cancer (LECC) who were not performed all stages of ESP, including the reconstructive one, the postoperative period was more difficult due to unsatisfactory immediate and long-term results. It should be noted that the advantage of the reconstruction method that we have developed, which is technically affordable and does not show an evident cosmetic defect, thereby providing more favorable conditions for postoperative rehabilitation of patients. Also, this method does not require the use of expensive tools and / or expensive surgical stapling instrument, suture material. The life quality in dynamics was higher in patients of the main group compared with the control one due to a decrease in postoperative complications, an improvement in the rehabilitation period as a result of the application of various methods of reconstructive and recovery operations after ESP.We can say that such a superradical surgical intervention as ESP requires a versatile, multidisciplinary approach, starting with careful selection of patients for surgery, preoperative preparation and the selection of the optimal type of anesthesia up to the staged surgery itself. The reconstructive stage of ESP is important because it allows to reduce and / or to prevent complications such as intestinal obstruction, small intestinal fistulas, recto-vaginal and vaginal fistulas, lymphocele, pelvic abscesses and the formation of pelvic hernias. The choice of the most optimal type of urine derivation and reconstruction of the pelvic floor after ESP largely determines the immediate and long-term results of the disease, reflecting the life quality of patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML