-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2020; 10(3): 141-146
doi:10.5923/j.ajmms.20201003.03

Liver as a Factor of Change of Gastrin Mechanisms of Regulation of Digestive Glands of the Stomach
Zhuraeva M. A. , Aleynik V. M. , Ashuraliyeva M. A.
Andijan State Medical Institute, Uzbekistan
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We studied the influence of factors affecting liver utilization of short-chain peptides as modifying mechanisms in the regulation of the digestive glands of the stomach. It was concluded that the short-chain peptide pentagastrin containing 5 amino acids is largely utilized by the liver, and the long-chain peptide gastrin-17 containing 17 amino acids is slightly utilized by the liver. Trypsin when passing through the liver reduces the utilization of pentagastrin by the liver, thereby stimulating the secretory function of the stomach. The protease inhibitor kontrakal during the passage through the liver increases the utilization ability of the liver pentagastrin, thereby reducing the secretory function of the stomach. The trypsin-inactivated pancreas and its inhibitors may be involved in the modification of the gastrin mechanisms of regulation of the digestive glands of the stomach.
Keywords: Liver, Utilization, Pentagastrin, Gastrin-17, Trypsin, Contrical, Rats, Gastric secretion, Pancreatic secretion
Cite this paper: Zhuraeva M. A. , Aleynik V. M. , Ashuraliyeva M. A. , Liver as a Factor of Change of Gastrin Mechanisms of Regulation of Digestive Glands of the Stomach, American Journal of Medicine and Medical Sciences, Vol. 10 No. 3, 2020, pp. 141-146. doi: 10.5923/j.ajmms.20201003.03.
- To date, in animals and humans, most peptides are present in more than one molecular form. At the same time, at least 10 molecular forms of peptides of the gastrin (G) group were revealed, containing in their structure from 4 to 56 amino acids, the physiological role of which has been little studied [15].The separation into short chain peptides and long chain peptides was carried out conditionally according to the results of studies where the physiological participation of the liver in increased utilization of short chain peptides containing up to 10 amino acids and low utilization of long chain peptides containing more than 10 amino acids was shown. Thus, influencing the regulation of the secretory, motor and neuromodulating functions of the digestive glands [2,10,11]. These data are consistent with the results of clinical studies, which demonstrate the presence of an excessive amount of circulating intestinal peptides that the diseased liver cannot utilize [8,13,14].Short-chain peptides have receptors on the afferent nerve endings of peripheral neurons and on neurons of various parts of the central nervous system. In the stomach and intestines, paracrine, they interconnect endocrine cells and neurons of the submucosal nerve plexus, mesenteric and afferent neurons. Thus, short-chain peptides are of great importance in the integration of various regulatory mechanisms.An increase in the production of short-chain peptides detected after food enters the gastrointestinal tract. In addition, short-chain peptides more efficiently stimulate the secretion of digestive glands and penetrate the blood-brain barrier. For example, due to CCK-8, a feeling of fullness is triggered, that is, a distant relationship between the cells of the digestive glands and various parts of the central nervous system is provided. This confirms the participation of short-chain peptides in the integration of peripheral and central mechanisms of regulation of the digestive glands.The utilization capability of the liver decreases in chronic liver diseases, due to which CCK-8 increases in the peripheral blood, as a result, encephalopathy can develop [11], as well as pancreatic hypersecretory syndrome [9,12] and gastric hyposecretory syndrome [13].In the supply of short-chain peptides to peripheral blood in the absence of physiological need, limiting mechanisms exist. So, part of short-chain peptides can be utilized in the intestine by intraorgan organ tissue and membrane proteases, and the other part in the liver, after entry through the portal system [1,10].The described mechanisms form additional channels of peptidergic regulation of the digestive glands.In recent years, in connection with the discovery of protease-activated receptors. It is suggested that pancreatic proteases should not be considered only from the traditional point of view as digestive enzymes, but additionally as signaling molecules that are actively involved in the spectrum of physiological and pathological conditions of both the gastrointestinal tract and other body systems. It is proposed that proteases as a whole be considered hormones, and the formation in this connection of new signaling pathways, as new regulation mechanisms in physiological conditions or new pathogenetic links in pathological conditions. [14].Previously, the participation of the liver in the utilization of short-chain peptide regulators (pentagastrin, leienkephalin, and CCK-8) was shown in our laboratory, which can be considered as an additional modifying factor in the peptidergic mechanisms of regulation of the digestive glands [4]. It was also established in our laboratory that, under the influence of intravenous administration of trypsin, the enzymatic excretory activity of the gastric glands increases [5].Objective: To study the influence of factors affecting the utilization of short-chain peptides by the liver as modifying mechanisms in the regulation of the digestive glands of the stomach.
1. Material and Methods
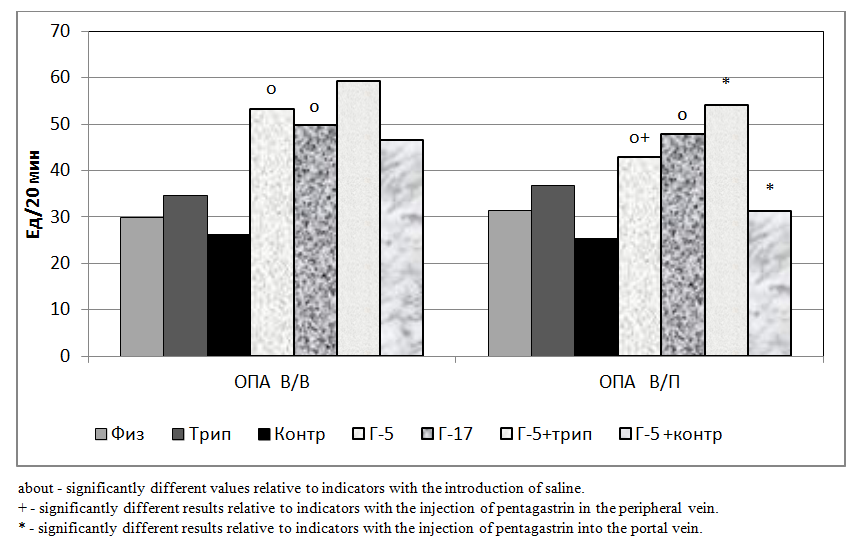
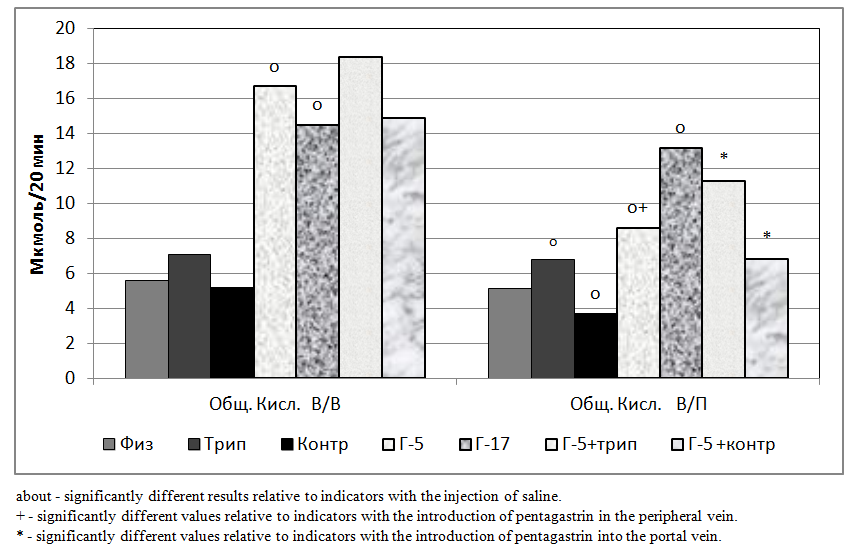
- The studies were carried out on 98 rats in 14 series, 7 acute experiments in each series. We studied the change in gastric secretion, in 1 series (control) when 0.3 ml of physiological solution was injected into the portal vein, in 2 series (control) when 0.3 ml of physiological solution was introduced into the peripheral vein.In the 3 series (experimental), a short-chain peptide - penta-gastrin (G-5) was injected into the portal vein at a dose of 0.1 μg / kg in 0.3 ml of physiological saline, in 4 series (experimental) - G- was introduced into the peripheral vein 5 at a dose of 0.1 μg / kg in 0.3 ml of physiological saline.In the 5th series (experimental), a long-chain peptide gastrin-17 (G-17) was introduced into the portal vein in an equimolar dose to pentagastrin of 0.28 μg / kg in 0.3 ml of physiological saline. In the 6th series (experimental), a long-chain peptide G-17 was introduced into the peripheral vein in an equimolar dose to pentagastrin 0.28 μg / kg in 0.3 ml of physiological saline.In the 7th of series (experimental), 0.3 ml of physiological saline was injected into the portal vein and, additionally, a contrical protease inhibitor (aprotinin) of 25,000 ATPE / kg was administered intraperitoneally.In the 8th series (experimental), 0.3 ml of physiological saline was injected into the peripheral and, additionally, a Contrical protease inhibitor (aprotinin) of 25,000 ATPE / kg was administered intraperitoneally. In the 9th series (experimental), G-5 was injected into the portal vein at a dose of 0.1 μg / kg in 0.3 ml of physiological saline and an additional contrical protease inhibitor (aprotinin) of 25,000 ATPE / kg was additionally administered intraperitoneally.In the 10th series (experimental), G-5 was injected into the peripheral vein at a dose of 0.1 μg / kg in 0.3 ml of physiological saline and a contrical protease inhibitor (aprotinin) of 25,000 ATPE / kg was additionally injected intraperitoneally.In series 11 (experimental), trypsin was injected into the portal vein at a dose of 300 μg / kg in 0.3 ml of physiological saline. In the 12th series (experimental), 0.3 ml of physiological saline was injected into peripheral trypsin at a dose of 300 μg / kg.In series 13 (experimental), G-5 was injected into the portal vein at a dose of 0.1 μg / kg, together with trypsin at a dose of 300 μg / kg in 0.3 ml of physiological saline. In series 14 (experimental), G-5 was injected into the peripheral vein at a dose of 0.1 μg / kg, together with trypsin at a dose of 300 μg / kg in 0.3 ml of physiological saline.The study was performed under hexenal anesthesia: 0.3 ml of a 5% solution of hexenal per 100 g of body weight was intraperitoneally administered. Gastric secretion was investigated by continuous perfusion according to Ghosh and Schild [6]. Gastric perfusate was collected for 20 minutes in periods of 40 minutes (two 20-minute periods) before and 40 minutes (two 20-minute periods) after administration of 0.1 mcg / kg penta-gastrin intraportally in 0.3 ml of physiological saline.As part of gastric perfusion solution, the following were determined: the isolation of proteases by total proteolytic activity (OPA) by spectrophotometric method [3,7], the hydrochloric acid rate by titration with NaOH perfusion solution [3,7].
2. Results and Its Discussion
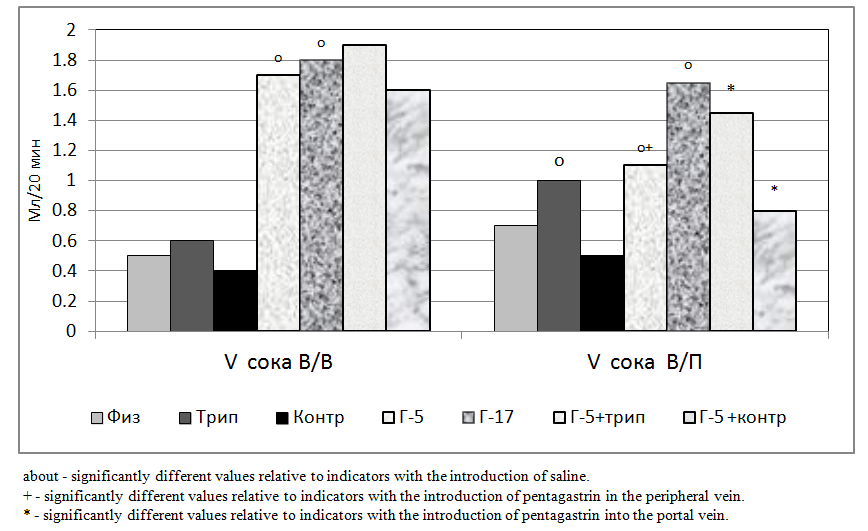
- The results of experiments on rats showed that the volume of secreted gastric juice under the influence of trypsin in the peripheral vein (iv) was not significantly higher than after administration of physiological saline. And under the influence of trypsin injected into the portal vein (iv), it was significantly higher than after the introduction of physiological saline (Fig. 1).At the same time, the volume of secreted gastric juice under the influence of contracal in the peripheral vein (iv) was not significantly lower than after the introduction of physiological saline. At the same time, under the influence of contracal introduced into the portal vein (iv), it was also not significantly lower than after the introduction of physiological saline, but this effect was more pronounced than when introduced into the peripheral vein (Fig. 1).The volume of secreted gastric juice under the influence of G-5 injected both into the peripheral vein and the portal vein was significantly higher than those after administration of physiological saline. Moreover, the parameters under the influence of G-5 injected into the portal vein were significantly lower than the parameters introduced into the peripheral vein. At the same time, under the influence of G-17, introduced into the peripheral vein (iv), the volume of secreted gastric juice was also significantly higher than after injections of saline and slightly higher than G-5 (Fig. 1). The intra-portal administration of G-17 caused a significant increase in the volume of gastric juice, as compared with the intra-portal administration of physiological saline and an unreliable increase in comparison with G-5.At the same time, the indices during intraportal administration of G-17 were slightly lower than when this peptide was introduced into the peripheral vein (Fig. 1).With the combined administration of trypsin and G-5, in relation to the results of the administration of G-5 alone, there was an unreliable increase in indicators when injected into the peripheral vein and a significant increase when introduced into the portal vein. At the same time, under the influence of co-contracal and G-5, there was an unreliable decrease in indicators when injected into the peripheral vein and a significant decrease when introduced into the portal vein (Fig. 1).
3. Conclusions
- The short-chain peptide pentagastrin is largely utilized by the liver, and the long-chain peptide gastrin-17 is slightly utilized by the liver. Trypsin, when passing through the liver, reduces the ability of the liver to utilize pentagastrin, thereby stimulating the secretory function of the stomach. The protease inhibitor contrikal when passing through the liver increases the ability of the liver to utilize pentagastrin, thereby reducing the secretory function of the stomach. Pancreatic hormone trypsin and its inhibitors can be involved in the modification of gastrin mechanisms of regulation of the digestive glands of the stomach.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML