-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(2): 503-508
doi:10.5923/j.ajmms.20190912.12

Comparative Evaluation of Efficiency of Hemopoetic Stem Cells Autotransplantation at Multiple Myeloma
Gulchekhra Zukhriddinovna Makhamadalievа1, Khamid Yakubovich Karimov2, Abdurakhmon Abdumavlyanovich Kayumov3
1Hematopoietic Stem Cell Treatment Unit, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
3Administration, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Gulchekhra Zukhriddinovna Makhamadalievа, Hematopoietic Stem Cell Treatment Unit, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Our study showed that positive results of testing for type G immunoglobulin (IgG) among patients with multiple myeloma (MM) in Uzbekistan are recorded 3.55 times more often than in the detection of type A immunoglobulins (IgA). In this case, the detection frequency of the light chains of kappa (κ) and lambda (λ) is 1.39 times higher when studying the secretion of IgG type than when studying the production of IgA. At the same time, the results of the treatment indicate that the percentage of recurrence free surviva (RFS) and overall survival (OS) of MM patients in subgroup “B” among patients who received polychemotherapy (PCT) in combination with autologous hematopoietic stem cell transplantation (HSCT), exceeds those in subgroup “A”, among patients receiving only PCT. This, in turn, indicates a higher efficiency of the use of autoHSCT, which is reflected in an increase in the quality of life and prolongation of both RFS and OS of MM patients in subgroup “B”.
Keywords: Multiple myeloma (ММ), Type G immunoglobulin (IgG), Type A immunoglobulins (IgA), Polychemotherapy (PCT), Autologous hematopoietic stem cell transplantation (auto-HSCT), Recurrence-free survival (RFS), Overall survival (OS)
Cite this paper: Gulchekhra Zukhriddinovna Makhamadalievа, Khamid Yakubovich Karimov, Abdurakhmon Abdumavlyanovich Kayumov, Comparative Evaluation of Efficiency of Hemopoetic Stem Cells Autotransplantation at Multiple Myeloma, American Journal of Medicine and Medical Sciences, Vol. 9 No. 2, 2019, pp. 503-508. doi: 10.5923/j.ajmms.20190912.12.
Article Outline
1. Introduction
- Multiple myeloma (MM) is a malignant tumor whose morphological substrate are plasma cells that produce monoclonal immunoglobulin. MM accounts for more than 10% of the total number of tumors of the hematopoietic system, and is characterized by a variety of forms, variants and clinical manifestations.Among MM variants, forms with secretion of various pathological immunoglobulins are distinguished. It should be noted that according to literature data, the most common forms of MM are secretions of IgG and IgA, the frequency of occurrence of which is 55–65% and 20–25%, respectively [1,3]. To date, it is known that the determination of the type of immunoglobulin is important both for the diagnosis and for the prognosis and further monitoring of the disease [8,10]. Along with this, the effectiveness of the treatment of multiple myeloma (MM) largely depends on the timely correct differential diagnosis of the disease variant [3]. The most common treatment for MM is the use of various protocols of polychemotherapy (PCT) [4]. However, the use of polychemotherapy does not always allow to achieve complete clinical and hematological remission, and thereby improve the quality of life and survival of patients with MM [7].Currently, in literature, you can find the results of a number of studies on the use of not only chemotherapy, but also autologous hematopoietic stem cell transplantation (autoHSCT) in patients with MM. AutoHSCT is one of the promising methods for treating patients with multiple myeloma (MM). The use of AutoHSCT significantly reduces the cell volume of the pathological clone and improves the quality of the response, thereby reducing the recurrence rate of multiple myeloma and increasing the overall survival (OS) of patients [4,5].
2. Main Body
2.1. The Purpose of Our Research
- Comparative evaluation of the effectiveness of autologous hematopoietic stem cell transplantation (autoHSCT) in patients with multiple myeloma (MM).
2.2. Material and Methods of Study
- The study included 107 patients (median age - 55.3 ± 2.3 years) with a diagnosis of multiple myeloma (MM), who were registered at the dispensary and treated in the period from 2013 to 2018. at the Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan (Uzbekistan, Tashkent).Of the total number of MM patients, men accounted for 48.6% (n = 52), and women 51.4% (n = 55).Verification of the diagnosis was carried out according to international diagnostic criteria: the results of a clinical examination, laboratory parameters and immunological variants of the production of immunoglobulins of types of heavy and light chains were taken into account [9]. An immunochemical study included electrophoresis with immunofixation of blood serum proteins using an analyzer from Interlab Pretty (Italy) using reagents of the same brand.Patients, depending on the method of treatment used, are divided into two groups: group 1 (n = 87) patients with MM who received 4 courses of PCT according to the VCD protocol (Bortezomib (PS-341) at 1.3 mg / m2 intravenously in 1, 4, 8 and 11 days; cyclophosphamide 300 mg / m2 intravenously on days 1, 8, 15 and dexamethasone 20 mg orally or intravenously on days 1-2, 4-5, 8-9, 11-12) and group 2 (n = 20), consisting of patients who received 4 courses of PCT according to the VCD + autoHSCT protocol.Statistical processing of the obtained data was performed using Student's test using the programs "Microsoft Office Excel" and "Biostatistics 4.03". The criterion of statistical reliability was p <0.05.
2.3. Results of the Study
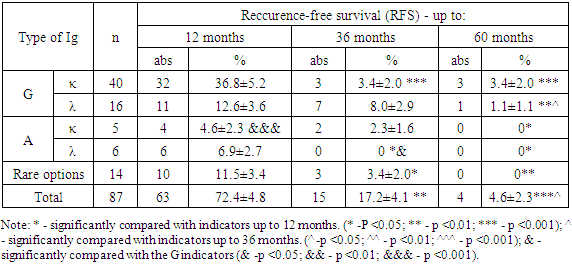
- During the study, all MM patients (n = 107) were tested for detectable immunoglobulins, of which IgG κ (kappa) -chain type immunoglobulins were found in 47 (43.9%), IgG λ (lambda) -chain in 24 (22, 4%), IgA κ (kappa) chains in 6 (5.6%), IgA λ (lambda) chains in 14 (13.08%). Additionally, rare types of immunoglobulins were determined in 4 (3.7%) patients: a combination of IgG-κ (kappa) and IgA-λ (lambda), in 1 (0.9%) a combination of IgG-λ and IgM-κ, in 2 ( 1.87%) of patients with a combination of IgGκ and free "light" λ chain and in 1 (0.9%) - IgАκ and free "light" chain λ. Along with this, 6 (5.6%) patients had free “light” λ chains and 1 (0.9%) κ chains.An analysis of the frequency of immunoglobulin types among MM patients showed that IgG is recorded 3.55 times (p <0.05) more often with respect to IgA. In this case, the detection frequency of the light chains of kappa (κ) and lambda (λ) is 1.39 times higher (p <0.05) with IgG secretion than with IgA type.With a median follow-up of 60 months (range 1–96 months), the duration of overall survival (OS) and reccurence free survival (RFS) in patients with multiple myeloma (MM) had some differences.In the “A” subgroup among patients with multiple myeloma (MM) who received only 4 courses of polychemotherapy (PCT) according to the VCD protocol (n = 87), reccurence-free survival (RFC) of up to 12 months was recorded in 63 patients, which averaged 72.4 ± 4.8% (p> 0.05), up to 36 months - at 15 (17.2 ± 4.1%; p <0.01) and up to 60 months and more - at 4 (4.6 ± 2, 3%; p <0.01 and p <0.05) (Table 1).
|
|
|
|
3. Conclusions
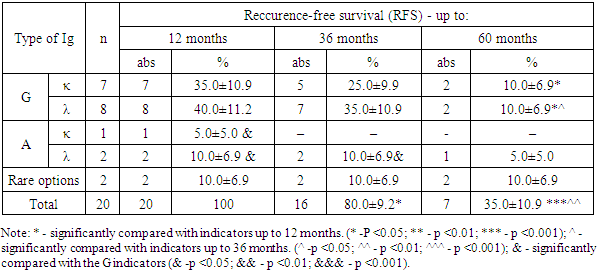
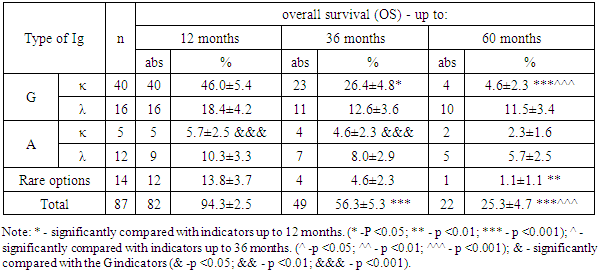
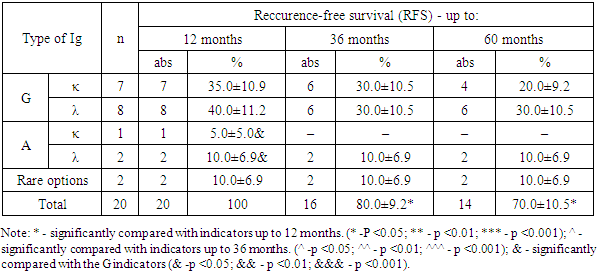
- The course of multiple myeloma in many respects depends on the immunochemical variant with the involvement of certain immunoglobulins of heavy and light chains. So, for example, A-myeloma, in comparison with G-myeloma, is accompanied by a more aggressive course, which proceeds with severe tumor intoxication, bone plasmacytomas, high proteinemia, calcium, urea, creatinine, γ- and β2-microglobulins in blood serum. Whereas D-myeloma is characterized by a high level of proliferation of myeloma plasma cells, production of β2-microglobulin, with the association of various genetic abnormalities [12].Our study showed that among patients with MM, the frequency of occurrence of types of IgG immunoglobulins, relative to IgA, is recorded 3.55 times more often. According to published data, the detection rate of G- and A-myeloma is 55-65% and 20-25%, respectively [3]. At the same time, the detection frequency of the light chains of kappa (κ) and lambda (λ) is 1.39 times higher with IgG secretion than with IgA type.Since the application of the first treatment regimens for multiple myeloma, its main goal has been to prolong the life of patients, which largely depends on a timely, correct diagnostic approach and the level of development of medical technologies.Literary sources indicate that prior to the use of alkylating drugs, the median overall survival of patients with multiple myeloma was only 17 months. The low life expectancy of patients was largely associated with frequent infectious complications, extramedullary lesions, severe renal failure, which were increasingly exacerbated by concomitant anemia and bone damage [8]. All this was the impetus for the study and search for new methods and approaches in the treatment of multiple myeloma [11,16,17].In recent years, the results of treatment have been accumulated with the introduction of hematopoietic stem cell transplantation (HSCT) into the treatment regimen of multiple myeloma, in particular, auto-HSCT. Numerous studies on the use of high-dose polychemotherapy (PCT) followed by auto-HSCT have shown their high efficiency among patients with recurrent and resistant forms of multiple myeloma [13,15,19,20].The literature provides evidence that Intergroupe Francophone du Myelome (IFM, 1990) first conducted a study comparing the results of standard polychemotherapy (PCT) and PCT + auto-HSCT in 200 patients with multiple myeloma (MM). The results of the study showed that in the group of patients using standard polycheotherapy (PCT) + auto-HSCT, significantly better results were obtained. Thus, the frequency of complete remission, DBS and OV were 4.4 (22% versus 5%), 1.6 (28 months versus 18 months) and 1.3 (median 57 months versus 44 months) times higher in relation to patients without auto-HSCT [5,18]. Research results Adam Z., Krejci M., Tichy M. et al. (2009) showed that the use of autoTGSC increases the frequency of complete remissions of disease-free survival (DFS) and overall survival (OS) in patients with multiple myeloma (MM).In our studies, the results of the treatment indicate that the percentage of patients with MM with ADV and OV in subgroup “B” who received polychemotherapy (PCT) in combination with auto-HSCT exceeds those in subgroup “A” of patients who received only PCT. This, in turn, is evidence of a higher efficiency of the use of auto-HSCT, which is expressed in improving the quality of life and prolonging both disease-free survival (DFS) and their overall survival (OS) in patients with multiple myeloma (MM) in subgroup “B” .Thus, multiple myeloma (MM) is a complex little-studied disease, manifested by a variety of clinical presentation and laboratory signs, which largely determine its course and prognosis. Achievements and implementation of new technologies in the field of diagnostics (determination of the immunochemical option) and treatment of MM (the use of targeted drugs in combination with autoTGSCs) contribute to the timely diagnosis of the disease, determine its prognosis and prescribe targeted treatment, which aims to improve the quality of life of patients by increasing the median relapse-free survival (DFS) and overall survival (OS).
ACKNOWLEDGEMENTS
- We express our gratitude to the doctor laboratory assistant of the Laboratory of Medical Genetics and the Department of Molecular Medicine and Cellular Technologies of the Research Institute of Hematology and Blood Transfusion for performing laboratory studies related to the analysis of the immunochemical type of multiple myeloma – Liya Kamilovna Mustafina.We are also grateful to the head of the laboratory of medical genetics of the Research Institute of Hematology and Blood Transfusion for the overall guidance of the the laboratory tests – Kodirjon Tukhtabaevich Boboev.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML