-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(2): 490-492
doi:10.5923/j.ajmms.20190912.09

The Use of Minimally Invasive Method of Removing Trumatic Subdural Hematomas
M. K. Agzamov , A. B. Tilyakov , I. M. Agzamov , F. G. Normurodov , R. M. Djalalov
Samarkand branch of Republican Research Centre of Emergency Medicine, Uzbekistan
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The analysis of surgical treatment of 41 patients operated on with acute subdural hematomas was carried out. In group 1 (27 observations), hematomas were removed by traditional craniotomy. In group 2 (14 observations), hematomas were removed using a new minimally invasive method. The results of surgical treatment depended on signs characterizing the severity of the patients’ condition: the degree of impaired consciousness, the volume of the hematoma, the degree of the brain median structures displacement. The use of open craniotomy is necessary in all cases when the level of the victims’ consciousness was lower than the sopor and the hematoma volume exceeded 60 cm³. In the case of violation of patients’ consciousness level not lower than deep torpor, the volume of the hematoma is not higher than 40-60 cm³ and the minimum severity of symptoms of lateral dislocation, when the displacement of the brain middle structures does not exceed 5 mm, a minimally invasive method of removing the subdural hematoma can be used.
Keywords: Traumatic subdural hematoma, Minimally invasive method
Cite this paper: M. K. Agzamov , A. B. Tilyakov , I. M. Agzamov , F. G. Normurodov , R. M. Djalalov , The Use of Minimally Invasive Method of Removing Trumatic Subdural Hematomas, American Journal of Medicine and Medical Sciences, Vol. 9 No. 2, 2019, pp. 490-492. doi: 10.5923/j.ajmms.20190912.09.
1. Introduction
- Subdural hematoma is a local accumulation of blood between the solid and arachnoid cerebral membranes and makes up about 40% of all intracranial hemorrhages. In most cases, the subdural hematoma is a consequence of a traumatic brain injury (TBI), and the frequency of its occurrence in severe TBI reaches 22% [1-2].The most common causes of traumatic subdural hematoma (TSH) formation are accumulation of blood around the area of brain injury, as well as rupture of superficial or passing to the dura mater vessels. The choice of treatment method for TSH is surgery. Preference is given to osteoplastic trepanations of the skull with removal of the subdural hematoma. Decompressive resection trepanations are performed at a rapid deterioration of patients’ condition [3-5]. In recent years, there are reports in the literature about the possibility of minimally invasive interventions for this pathology [1,3]. Under the certain circumstances, such as a compensated condition, the absence of dislocation signs, the presence of a certain volume of hematoma, it is impractical to carry out wide osteoplastic trepanations. Thanks to the use of MSCT, it became possible to determine the quantitative (size, volume) characteristics of the hematoma, the timing of its formation, localization, as well as the degree of its effect on the brain and, as a result, the possibility of dynamic monitoring of intracranial pathology in general and hematomas in particular [6-7]. In this regard, the interest in the use of less traumatic surgical methods for removing TSH, the definition of indications and contraindications for their use are understandable.Aim of the research is to optimize the surgical treatment results of traumatic subdural hematomas.
2. Materials and Methods
- The surgical treatment analysis of 41 patients with traumatic subdural hematomas was carried out. Patients have been treated in the Samarkand branch of the Republican Research Center of Emergency Medicine from 2017 to 2019. There were 7 patients under the age of 18 years and 34 patients over 18 years. There were 31 men, 10 women (Fig. 1).
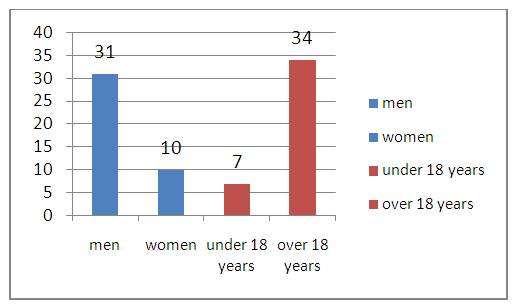 | Figure 1. Distribution of patients by gender and age |
 | Figure 2. The consciousness level of the investigated patients |
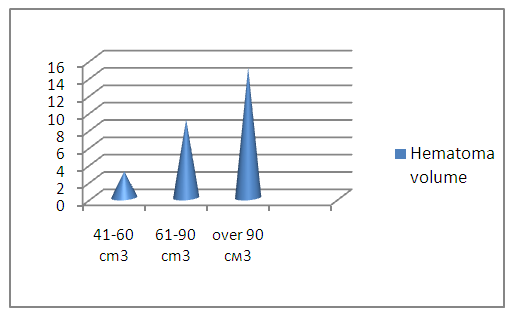 | Figure 3. The hematoma volume of the investigated patients |
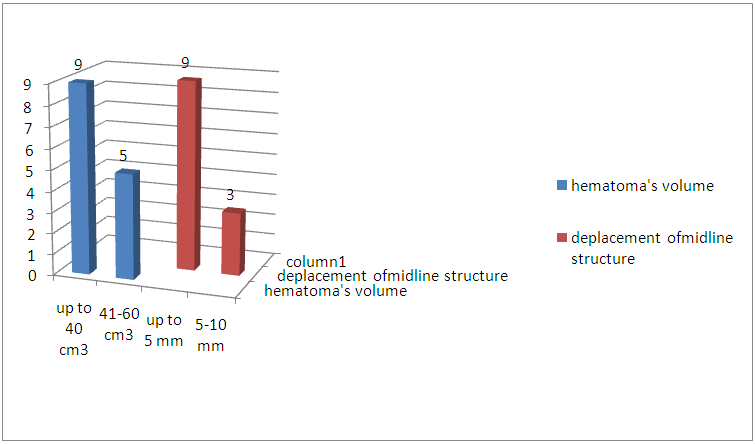 | Figure 4. The volume of hematoma and the displacement of the median structures |
3. Results
- The treatment results of patients were evaluated according to the hospital mortality rate and types of functional outcomes, for which the Glasgow outcome scale was used. We analyzed the disease outcomes depending on the severity of the patients’ condition, the level of impaired consciousness, the amount of hemorrhage and the brain median structures displacement. The level of consciousness disorder and the severity of the condition were determinative in the estimation of severity and significantly influenced on the disease outcome. So, from 5 patients who arrived in clear consciousness there were no dead. From the 8 patients admitted in torpor one died; from 12 in sopor - 2, from 16 in a coma – 6 died. (Fig. 5.)
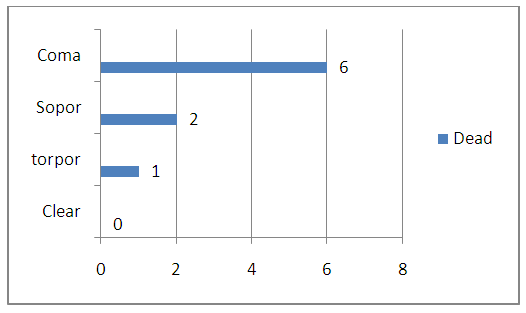 | Figure 5. The lethality rate by consciousness level |
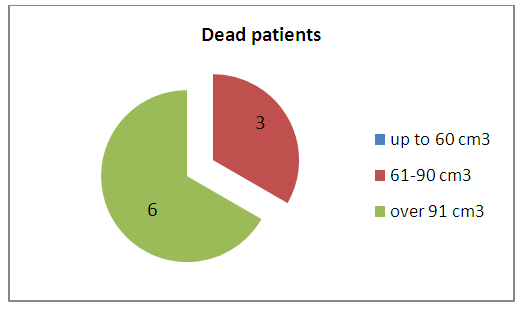 | Figure 6. The lethality rate depending on hematoma level |
4. Discussion
- The results of surgical treatment in groups depended on signs characterizing the severity of the patients’ condition — the degree of impaired consciousness, the volume of the hematoma, the degree of the brain median structures displacement. The use of open craniotomy was necessary in cases when the level of consciousness in patients was lower than the sopor and the hematoma volume was above 60 cm³. Emergency decompression was carried out as an element of resuscitation in cases of an increase in the dislocation syndrome. The data obtained showed that the use of a minimally invasive method for removing hematoma is possible subject to a number of conditions:• level of patients impaired consciousness not lower than deep torpor;• the volume of the hematoma is not higher than 40-60 cm³;• minimal severity of lateral dislocation symptoms when the displacement of the brain median structures does not exceed 5 mm.Encouraging clinical results were obtained subject to the above mentioned conditions. The volume of the removed hematoma was insufficient in 2 from 17 patients and therefore they needed repeated surgical interventions – bone-plastic craniotomy. The bone cutting lines were performed through previously superimposed milling holes. The application of this method should be strictly controlled by clinical, neurological and neuroimaging methods in the postoperative period.
5. Conclusions
- The use of a minimally invasive method when removing traumatic subdural hematomas is possible subject to certain clinical, neurological and neuroimaging conditions and their control in the postoperative period. The low-invasiveness of the method in combination with the short duration of the operation and the results obtained in this way allow further accumulation of data to study the indications and contraindications for its use.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML