-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(2): 464-466
doi:10.5923/j.ajmms.20190912.03

Association of Gene Polymorphism Adipoq +276 (G / T) with Surrogatic Markers of the Increased Fat Content in the Liver
Iminova D. A., Alyavi A. L., Sobirova G. N.
Republican Specialized Scientific, Practical Medical Center of Therapy and Medical Rehabilitation, Tashkent, Uzbekistan
Correspondence to: Iminova D. A., Republican Specialized Scientific, Practical Medical Center of Therapy and Medical Rehabilitation, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents the results of clinical, biochemical and genetic studies of 80 patients with non-alcoholic fatty liver disease (NAFLD) of the patients with uzbek nationality. It showed the associations between the gene polymorphism and ADIPOQ with dyslipidemia, in the group of patients with NAFLD, especially in women with elevated BMI.
Keywords: Adiponectin, ADIPOQ, Single nonalcoholic fatty liver diseases, Dyslipidemia
Cite this paper: Iminova D. A., Alyavi A. L., Sobirova G. N., Association of Gene Polymorphism Adipoq +276 (G / T) with Surrogatic Markers of the Increased Fat Content in the Liver, American Journal of Medicine and Medical Sciences, Vol. 9 No. 2, 2019, pp. 464-466. doi: 10.5923/j.ajmms.20190912.03.
1. Introduction
- Non-alcoholic fatty liver disease (NAFLD) is considered to have a wide range of forms of liver damage, ranging from hepatocellular steatosis, steatohepatitis, fibrosis to liver cirrhosis and hepatocellular carcinoma. The prevalence of NAFLD has an association with an increase in parallel with the frequency of obesity and diabetes, occupying a leading place among other etiological forms of chronic liver diseases in Western countries. NAFLD is currently regarded as one of the main causes of the development of cryptogenic cirrhosis. The prevalence of NAFLD varies between 20 and 30% and reaches 90% in obese patients [1,2].Recently researchers have begun to pay attention to genetic markers in various pathologies [3]. At present time there have been studied the several gene candidates for the development and progression of NAFLD [4]. The product of the gene ADIPOQ (adiponectin) is a hormone that is synthesized and secreted by a white adipose tissue (AT) (predominantly adipocytes of the visceral region). With obesity, there is a decrease in the activity and amount of adiponectin in the blood. Adiponectin has been shown to inhibit the differentiation of preadipocytes, which confirms its possible effect on the regulation of adipose tissue. The level of adiponectin in the blood plasma is inversely proportional to the mass of AT and an indicator of WV / VH ratio (waist volume to the volume of the hips) [5]. The adiponectin levels are reduced with obesity, in contrast to other adipokines, which are increased, including leptin, resistin, and tumor necrosis factor-α (TNF-α). It is assumed that the development of insulin independent diabetes (type 2) may be associated with dysregulation of adiponectin secretion. The decrease in adiponectin expression has been shown to correlate with insulin resistance (IR) and that adiponectin is associated with glucose metabolism. Addition of recombinant adiponectin shows an inhibition of the glucose synthesis in the liver. Adiponectin is believed to have a protective function against hyperglycemia, IR and atherosclerosis, at least by partial antagonizing the activity of TNF-α [6,7]. One of the most relevant SNPs at the ADIPOQ locus are a G to T substitution in intron 2 (+ 276 G>T, rs1501299). The G allele has been negatively [8] and positively [7] associated with obesity in some populations. In addition, this SNP has been associated either with increased or decreased concentrations of plasma adiponectin [9,10]. On the other hand, this genetic variant has been negatively [11] and positively [12] associated with obesity in some populations. Another area with uncertain results is the relationship of this genetic variant to glucose metabolism and IR [13,14].The aim of our study was to evaluate the effect of gene polymorphism ADIPOQ +276 (G/T) rs1501299 on lipid spectrum of patients with NAFLD of Uzbek nationality.
2. Material and Methods
- The study included 80 patients aged from 22 to 60 years with NAFLD, who underwent the treatment at the Republican Specialized Scientific-Practical Medical Center of Therapy and Rehabilitation of the Ministry of Health of the Republic of Uzbekistan. The control group constituted 30 healthy, age-matched, randomly selected persons. The diagnosis of NAFLD was established on the basis of clinical history, clinical examination, laboratory tests and liver ultrasound. All patients consumed less than 30/20 g / day for men / women ethanol showed no signs of chronic viral B, C, D, hepatitis; autoimmune hepatitis and drug-induced hepatitis, Wilson’s disease, idiopathic hemochromatosis, α1-antitrypsin deficiency. Study was conducted in accordance with the guidelines of the Helsinki Declaration of the World Medical Association's "Ethical Principles for Medical Research Involving Human Subjects" with amendments (2013). All patients who participated in this study gave written informed consent and the protocol was approved by the National Ethics Committee of Uzbekistan. Clinical and biochemical monitoring was performed for all patients. The investigation of ADIPOQ gene polymorphism was performed in the laboratory of the Center of advanced technologies on the basis of the Ministry of innovative development of the Republic of Uzbekistan. DNA samples were isolated from peripheral blood leucocytes by using DNA extraction kit Diatom™ DNA Prep 200 (“IsoGen Laboratory”, Moscow, Russia).
|
|
3. Research Results
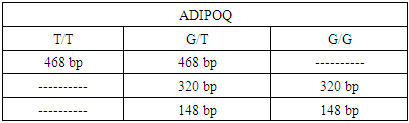
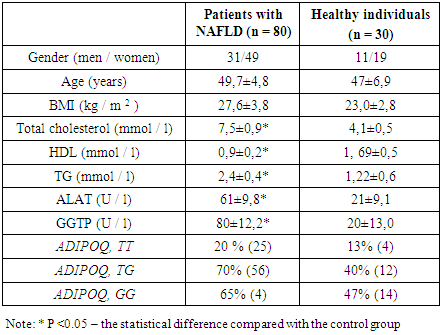
- After genotyping of all the individuals, the presence of genotypes TT, GT, GG and ADIPOQ gene was revealed. Genotype frequencies of ADIPOQ +276 (G/T) rs1501299 polymorphism in patients with NAFLD and controls are shown in table 3. The genotype distributions of the ADIPOQ +276(G/T) polymorphisms were in Hardy–Weinberg equilibrium in control groups controls (p>0.05). Comparanive analysis of resulting gtnotypes between patients and controls showed significant association between TG genotype and NAFLD assuming an additive model (p=0.03, Cochran-Armitage trend test) and recessive model (p=0.02, Pearson’s χ2 test). Conducted investigations have identified the increased body mass index (BMI), aminotransferase, dyslipidemia in patients having NAFLD compared to the healthy persons.
|
4. Conclusions
- In this way the conducted studies of this section showed that patients with NAFLD have increased incidence of the T allele of ADIPOQ +276(G/T) gene polymorphism rs1501299 compared to the healthy individuals. It was also noted that the presence of the T allele was a marker of severity of lipid spectrum disorders (hypercholesterolemia and increased triglyceride levels, an increase in the BMI level. Effects of pathological polymorphism on indicators of cytolysis and cholestasis were expressed to a lesser extent.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML