Shamsiev Azamat Muxitdinovich1, Qodirov Nizom Daminovich2
1Department of Pediatric Surgery, Doctor of Medical Sciences, Samarkand State Medical Institute, Uzbekistan
2Department of Pediatric Surgery, Samarkand State Medical Institute, Uzbekistan
Correspondence to: Qodirov Nizom Daminovich, Department of Pediatric Surgery, Samarkand State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Varicocele - pathological expansion and tortuosity of the scrotum. The term Varicocele refers mainly to the expansion and tortuosity of the components of the venous plexus draining the testicles. This plexus is located on the back of the testicles and epididymis. The morphological method is one of the most objective in assessing the state of varicose and convoluted veins with Varicocele. Knowledge of the morphological status of varicose veins of the scrotum can serve as a determining factor in choosing the method of surgical intervention. However, there is a small number of studies of structural changes in v spermatica with Varicocele. To study varicose veinsdilated seminal veins, their fragments were obtained during surgical excision of persons aged 15 to 20 years. Samples were fixed in a 10-12% solution of neutral formalin. After appropriate treatment, the pieces were poured into paraffin and sections were prepared with a thickness of 5-7 μm. The general morphological picture was studied on sections stained with hematoxylin and eosin. For scanning electron microscopy after fixation preparations in 2.5% solution of glutaric aldehyde in phosphate buffer, dehydrated in an alcohol-acetone. The studied veins of the void (pear-shaped) plexus (pampiniform venous plexus) of the spermatic cord are of the size of medium-sized veins. The interweaving of smooth muscle fibers is especially pronounced at the points of contact of the vein walls in the plexus. In these sections, t is not determined. Adventitia. For interwoven branches of the veins of the plexus t. Adventitia forms a common layer - the bed for the entire plexus, this layer passes into a kind of thick connective tissue case with large layers of adipose tissue. Among the identified accumulations of blood cells, erythrocytes dominate, and their pathological forms are echinocytes. Identified features should be considered when conducting surgical treatment of Varicocele.
Keywords:
Varicocele, Varicose dilated seminal veins, Structural features, Morphological picture, Scanning electron microscopy
Cite this paper: Shamsiev Azamat Muxitdinovich, Qodirov Nizom Daminovich, Structural Features of the Seminal Veins with Varicocele, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 445-452. doi: 10.5923/j.ajmms.20190911.09.
1. Relevance of the Research Problem
Varicocele is a pathological dilatation of the spermatic cord venous plexus, found in 15–20% of adult men and up to 40% of not mature boys [1,6]. This pathology is more often observed on the left side of the scrotum and may cause a decrease in testicular (sexual) function [11,13,15]. There are several theories of the etiology of this dysfunction. The main thing is the anatomical features of the venous plexus, including the angle of incidence of the testicular vein into the left renal vein, which determine the functional insufficiency of the venous valves at the junction of the testicular and renal veins [8,9,12,14].According to the size of the diameter and thickness of the walls of the veins, there are: small veins - venules (postcapillary and muscle), medium-sized veins and large veins. The structural features of the walls determine that the lumens of the veins, unlike arteries, are collapsed. Because of this, it is impossible to accurately determine the area of the lumen of the veins.Three walls are distinguishable in the walls of the medium and large veins: tunica intima, t. media, t. adventitia [15,18].Large veins and medium-sized veins, as a rule, accompany the corresponding arteries. The walls of the veins are thinner than the walls of the corresponding arteries, and the gaps are larger [8,12].Among the various pathological changes in the veins, the most common is the expansion of their lumen. They come in the form of a simple extension (phlebectasia) and varicose veins.Phlebectasia is a common, uniform expansion of the veins, not accompanied by a violation of the structure of their walls. After the stopping effect of stagnation of blood, phlebectases are reduced. This is often observed with Varicocele I degree, which often passes with age and normal regulation of sexual activity.Varicose veins are the progression of phlebectasia. In this case, there is an increase in the diameter and lumen of the veins. The expansion of the veins becomes uneven, with nodular protrusions of the walls. The process usually progresses, changes in the structure of the veins are exacerbated, combined with a disorder of blood circulation in them and thrombosis.There are three main types of varicose veins: cylindrical, serpentine and saccular. With severe disease, a mixed form of varicose veins occurs. With varicose veins of the spermatic cord, cylindrical and serpentine forms most often develop [4,20].The expansion of the veins of the spermatic cord is accompanied by changes in all three shells of their walls.As noted above, Varicocele is a pathological expansion and tortuosity of the scrotum. The term Varicocele refers mainly to the expansion and tortuosity of the components of the venous plexus draining the testicles. This plexus located on the rear surface of the testes and epididymides (epididymis) vas deferens. It connects to the genital or testicular veins. The right genital (right gonadal) vein flows into the superior vena cava system. The left one flows into the left renal vein at a right angle. The renal vein flows into the inferior vena cava [6,12,14].Small veins form plexuses in the form of a vine. Their sizes are 0.5-2.5 mm in diameter Dilation of these vessels with Varicocele can reach more 2 cm [2,6,12].Although it is noted that Varicocele is a bilateral disease [3,8,10].More than 90% of Varicocele are detected on the left, which is due to the anatomical difference between the left and right testicular venous vessels. The left testicular vein, as noted, flows into the left renal vein perpendicularly, the right v. testicular enters vena cava at an angle. This difference also determines that, left v. the testicular vein is longer than the right one and has a higher hydrostatic pressure [4,13].With Varicocele, structural changes are noted in all shells. Hypertrophy of muscle elements and intermuscular connective tissue is noted in the middle membrane. In those places where the inner shell has changes in the form of plaques, the middle shell is especially thin. In the cluster-like plexus, in the areas of contact between the walls of the vessels with each other, there is a strong development of longitudinal-muscular elements [4,12].A histological examination of the resected sections of the veins revealed sclerotic changes, both from the intima and muscle layers [6,11,14].The outer shell takes part in the process of changes in smooth muscle and connective tissue fibers. These fibers are almost always hyperplastic and, quite often, achieve significant development and, in their thickness, are much larger than the circular layer of the vessel.The listed changes are most pronounced near the testicle, they are weakly expressed in the inguinal canal and, starting from the inner opening of the inguinal canal upwards, are completely absent. Sometimes blood clots and phlebolitis can be found in the veins [6,11].A number of studies have shown a decrease in the number or even absence of venous valves in v. spermatica [1,6]. This leads to the fact that venous return can lead to expansion v. spermatica. Extension v. spermatica can be determined using the Valsalva method and ultrasound to determine the diameter of the vein [4,10,16].Currently, there are options for choosing various methods of surgical treatment. Despite the rather numerous studies of Varicocele, the mechanism of this pathology remains not completely clear. This also determines the emergence of controversial issues of surgical treatment of this pathology [7,10].The advent of laparoscopy has provided an alternative, more gentle access method in the treatment of many surgical diseases. The benefits of laparoscopic access are obvious. Less pain, a faster return to a full active life, a smaller postoperative scar. However, there are certain disadvantages of laparoscopic operations, among them the need for pneumoperitoneum (introducing gas into the abdominal cavity), mandatory general anesthesia with controlled breathing (endotracheal anesthesia), and more prolonged surgical intervention (together with anesthesia for 30-60 minutes) by experienced specialists. All this determines the higher cost of laparoscopic surgery.With laparoscopy, in particular with insufficient doctor experience, there is a risk of perforation or coagulation of the intestine, serious damage to the vessels and even accidental ligation of the ureter, which can be mistaken for the testicular vein.Gains distribution and angiographic embolization of the affected veins. To minimize the invasiveness of the operation, a method was proposed using angiographic transgenic embolization of Varicocele (testicular veins) [4,9,20].However, to date, in most states, open surgery remains the standard treatment for varicocele [1,8,18].For successful open operations using microsurgical technologies to determine the optimal options for intravenous anastomoses, a comparative morphological assessment is required, including the determination of various morphometric parameters of the veins with which the intravenous anastomoses are formed [17,19].The morphological method is one of the most objective in assessing the state of varicose and convoluted veins with Varicocele. Knowledge of the morphological status of varicose veins of the scrotum can serve as a determining factor in choosing the method of surgical intervention. However, there is a small number of studies of structural changes in v spermatica with varicocele.Morphological studies have shown that the rod-shaped venous plexus consists of veins of various diameters and rather different structures of the venous wall [5,9,11,12].However, detailed studies of v. spermatica with Varicocele was not performed. The three-dimensional structural organization of these veins has not been studied using scanning electron microscopy.This led to a true histological examination of various veins using morphometry.
2. Material and Research Methods
To study varicose veins of the seminal veins, their fragments were obtained during surgical excision of persons aged 15 to 20 years. Samples were fixed in a 10-12% solution of neutral formalin. After appropriate treatment, the pieces were poured into paraffin and sections were prepared with a thickness of 5-7 μm. The general morphological picture was studied on sections stained with hematoxylin and eosin (H&E).Study drugs and photographing was conducted using a microscope Axioscope (Carl Zeiss) with digital camera ProgRess, CapturePro 2.6, conjugate with the computer Pentium IV.For scanning electron microscopy (SEM) preparations after fixation in 2.5% solution of glutaric aldehyde in phosphate buffer, dehydrated in an alcohol-acetone, then dried by the critical point in the device 2 and HCP-sputtered gold in IB-2 apparatus. Viewing was carried out under a Hitachi S-405A microscope (Japan). Photographing was carried out from a monitor using a Canon digital camera. Morphological studies were conducted in the pathologic anatomy laboratory of the Republican Specialized Center of Surgery named after academician V. Vakhidov.Computer processing of the obtained video data was carried out using Windows Professional applications on a Pentium IV computer.
3. Research Results
The studied veins of the void (pear-shaped) plexus (pampiniform venous plexus) of the spermatic cord are of the size of medium-sized veins.In them, all three shells are quite distinctly distinguishable: tunica intima, t. media, t. adventitia (Fig. 1-6).A characteristic feature of this formation is the close contact of the walls of the vessels that form the specified cord (Fig. 1-3). Another feature of the plexus veins is rather narrow, partially collapsed gaps, the longitudinal dimensions of which are sometimes several times the size of the diameter. Blood clots and mixed blood clots, which are often intimately soldered to intima, are often detected in the lumen of veins (Fig. 1, 2). This layer is characterized by both flat endothelial cells, uniformly lining the surface, and protruding into the lumen of the cell, taking a cylindrical shape. Detachment of endothelial cell layers, their desquamation into the lumen (Fig. 3, 5-12, 20) is noted.The next characteristic feature of the studied veins is the corrugation of the inner (luminal) surface of tunica intima, which is due to its folding. Moreover, there are large folds that form the stellate lumen, and small folds that determine the internal surface relief (Fig. 2, 3, 5, 6).In t. media, the main structural formations are smooth muscles, located both longitudinally - in the form of a thin layer adjacent to the intimate, and a thicker circular, located - closer to t. adventitia (Fig. 5, 6).For varicose advanced testicular vein characteristic chaotic weave as numerous smooth muscle, and relatively few connective tissue fibers (Fig. 3, 5).Collagen fibers in the walls of the veins are distributed unevenly between muscle cells. They are often located in clusters of fibers of different sizes in all layers with a predominance in the inner longitudinal and circular layers or, forming cases around bundles of smooth muscle cells (Fig. 5, 6).The interweaving of smooth muscle fibers is especially pronounced at the points of contact of the vein walls in the plexus. In these sections, t is not determined. Adventitia. For interwoven branches of the veins of the plexus t. Adventitia forms a common layer - the bed for the entire plexus, this layer passes into a kind of thick connective tissue case with large layers of adipose tissue (Fig. 1-3).A characteristic feature of the walls of the plexus veins is the appearance between t. Media and t. Adventitia longitudinally spaced bunches of smooth muscle cells (Fig. 1, 4, 10, 12). These bundles are separated from each other by layers of loose connective tissue, of which t is composed. Adventitia. formed by loose connective tissue with significant spaces between the fibers and other structural formations, often, the zone of these bundles is thicker than t. Media (Fig. 4, 10, 12).T. adventitia is the most uneven layer in thickness (4–9). It is formed by loose connective tissue with wide layers of adipose tissue between the fibers, vessels, and other structural components (Fig. 1, 2, 4, 12).Studies have shown that the wall thickness of the studied plexus veins with varicose veins in different areas is not the same (1-3, 9).With varicose expansion, the wall thickness of the veins of the plexus tends to decrease, and the diameter of the vessels, both in the narrowest and widest places, increases. At the same time, the gaps of varicose veins often take more regular round-oval forms (Fig. 10.11).With a pronounced thinning of the wall of the varicose vein in some sections of tunica intima, peculiar thickenings form in the form of plaques penetrating the thickness of the middle layer (Figs. 1, 3).These plaques are lined, on the lumen side, by large endotheliocytes with rounded nuclei. Smooth muscle cells located perpendicular to the lumen of the vein are determined in their thickness. Often, speaking in the lumen, plaques can partially overlap it (Fig. 3).Scanning electron microscopy of the veins of the papilliform (pear-shaped) plexus (pampiniform venous plexus) spermatic cord showed that the most developed layer is t. Media (Fig. 13-16).T. intima in the investigated veins is quite polymorphic. Along with small folds lined with flattened endothelial cells (Fig. 13, 18, 19), there are areas lined with higher endothelial cells of a cubic and even prismatic shape (Fig. 17).In t. Media smooth muscle and, in part, connective tissue fibers rather randomly interwoven with each other, still have a distinct circular arrangement (Fig. 13-16).The main relief of the inner surface of the veins is determined by large folds and ridges, on the surface of which smaller folds are formed. The latter, as noted above, are lined with endothelium (Fig. 18). One of the characteristic features of the inner surface of the intima is the accumulation in the deep grooves of blood cells, mainly red blood cells and strands of fibrin (Fig. 20-23). Red blood cells and other blood cells may be located on the surface of the endothelial lining, but more often they are determined in areas where the endothelium is desquamated (Fig. 24-25).With large increases, it can be seen that among the red blood cells located on the inner surface of the vessels, in areas both lined with endothelium and, more often, deprived of the lining, along with discocytes, there are many pathological forms of red blood cells. Among pathological forms, echinocytes dominate - red blood cells with numerous processes (Fig. 26-28).The accumulation of red blood cells and other blood cells with strands of fibrin is the initial stage of the formation of blood clots. As noted above, when the walls of the veins merge in the plexus, t is not determined in the fusion zone. Adventitia. In the general connective tissue case into which t. Adventitia, a large number of fat cells are located.In SEM studies, accumulations of fat cells resemble clusters of grapes. They are interspersed with layers of loose connective tissue (Fig. 28).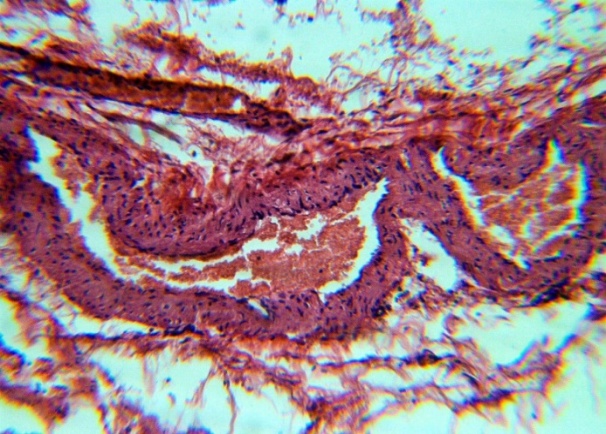 | Figure 1. The fusion of the walls of the veins of the plexus thrombi lumen. H&E. 10×10 |
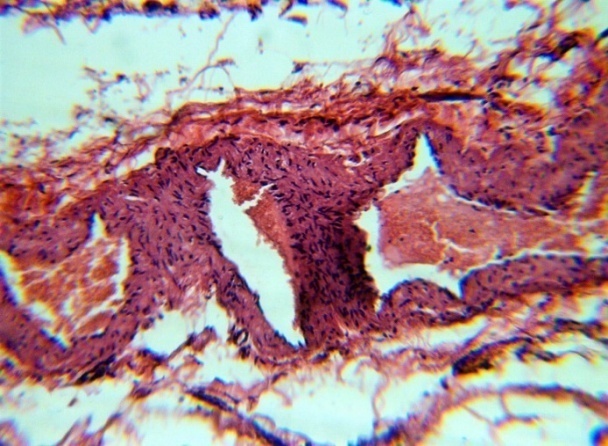 | Figure 2. The fusion of the walls of the veins of the plexus thrombi lumen. H&E. 10×10 |
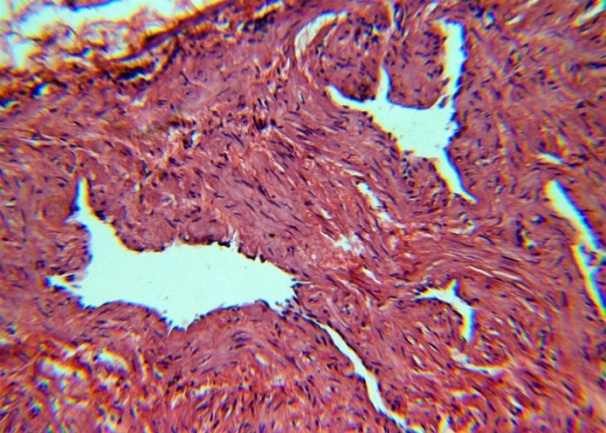 | Figure 3. The chaotic interweaving of smooth muscle fibers in t. Media. H&E. 10×10 |
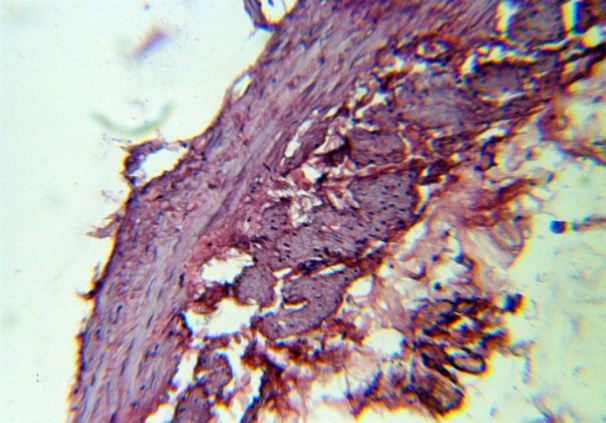 | Figure 4. Thinning t. Media, the appearance of bundles of longitudinal smooth muscle fibers between t. Media and t. Adventitia. H&E. 10×10 |
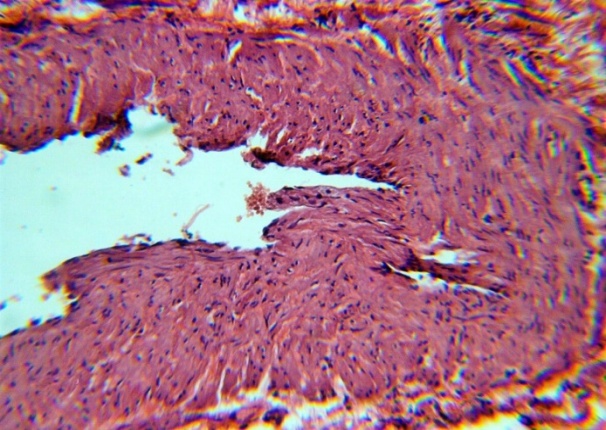 | Figure 5. Detachment of the endothelium, a layer of connective tissue fibers between smooth muscle. H&E. 10×10 |
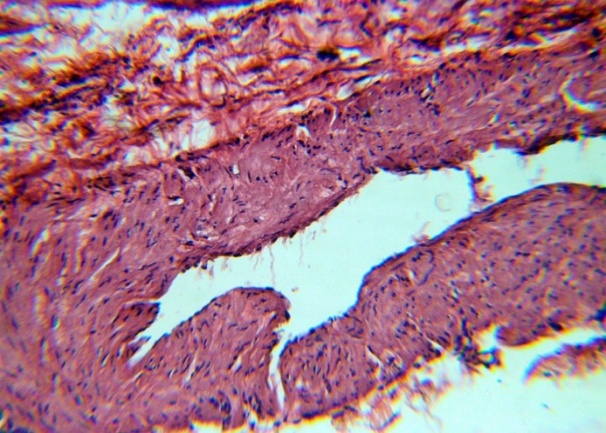 | Figure 6. Folds of the inner surface of the vein. H&E. 10×10 |
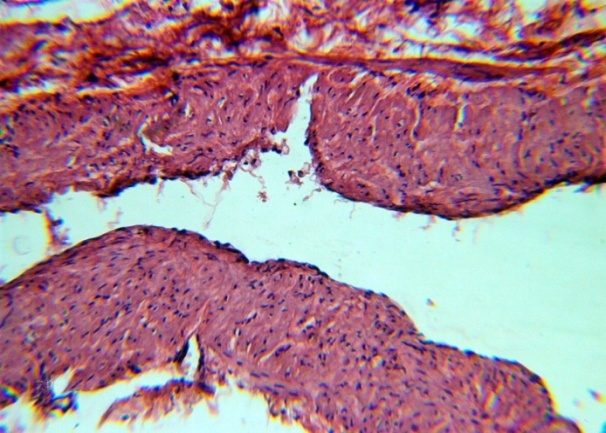 | Figure 7. Venous lumen, desquamation of the endothelium, interlayers of connective tissue fibers between smooth muscle. H&E. 10×10 |
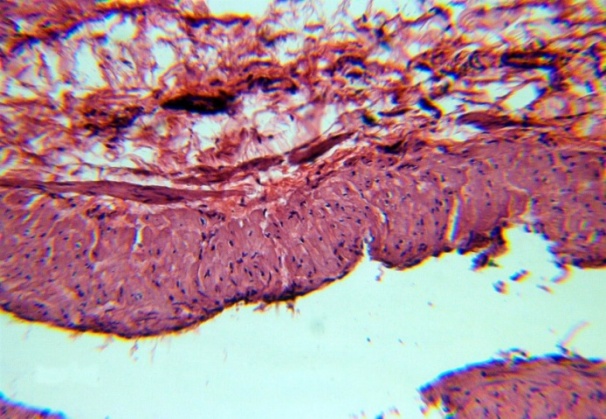 | Figure 8. Thickening t. Adventitia. H&E. 10×10 |
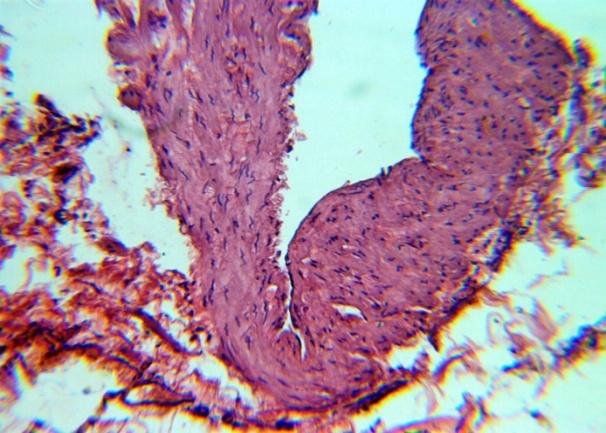 | Figure 9. Endothelial polymorphism, uneven thickness t. Media. H&E. 10×10 |
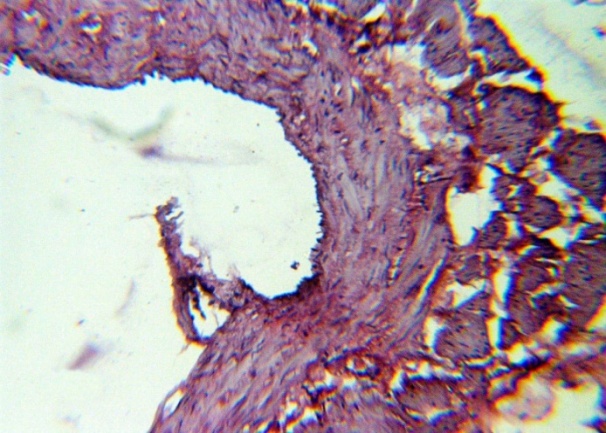 | Figure 10. The appearance of bundles of longitudinal smooth muscle fibers between t. Media and t. Adventitia. H&E. 10×10 |
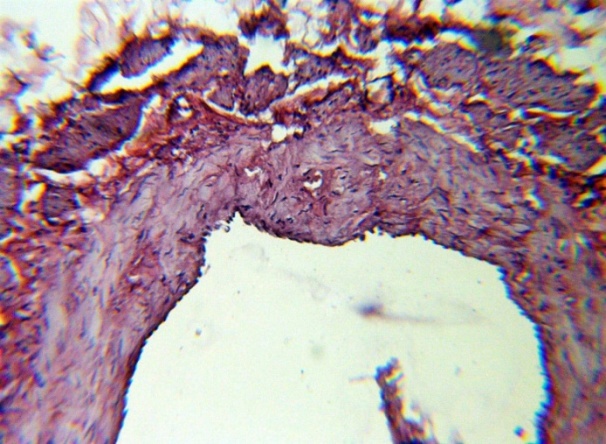 | Figure 11. The expansion of the lumen of the vein, longitudinal smooth muscle fibers between t. Media and t. Adventitia. H&E. 10×10 |
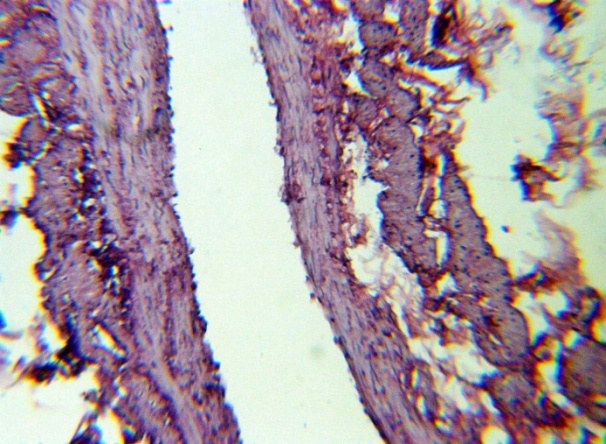 | Figure 12. Longitudinal smooth muscle fibers between t. Media and t. Adventitia. H&E. 10×10 |
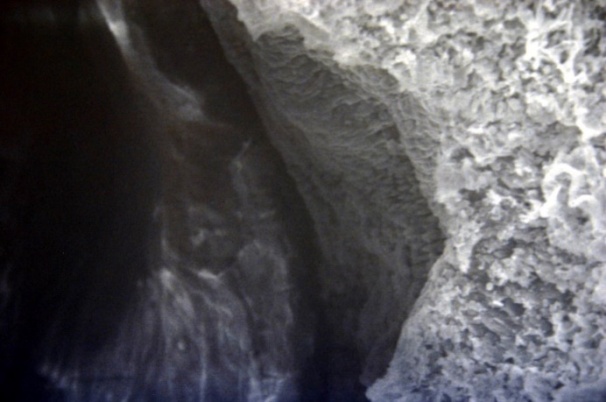 | Figure 13. Clearance of the veins. Inner surface with small folds SEM×100 |
 | Figure 14. Clearance of the veins. Small creased inner surface, smooth muscle weave t. Media. SEM×100 |
 | Figure 15. The interweaving of smooth muscle fibers t. Media is their circular orientation. SEM×100 |
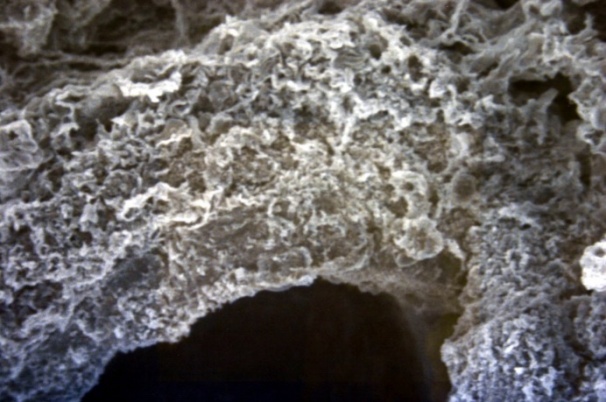 | Figure 16. The circular orientation. SEM×100 |
 | Figure 17. Endothelial cells of the inner surface of the prismatic form. SEM×400 |
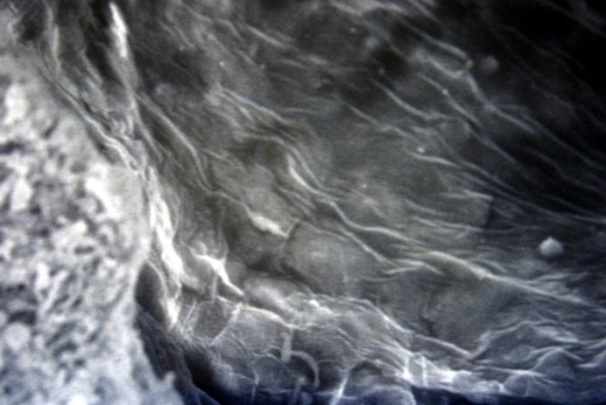 | Figure 18. The undulating surface of the intima with grooves and indentations. SEM×1000 |
 | Figure 19. Furrows and ridges of the inner surface of the intima. SEM×1000. |
 | Figure 20. Large ridges of the inner surface with accumulations of blood cells. SEM×400 |
 | Figure 21. Large ridges of the inner surface with accumulations of blood cells. SEM×400 |
 | Figure 22. The accumulation of blood cells in the recesses of the inner surface. SEM×1000 |
 | Figure 23. The accumulation of strands of fibrin and blood cells in the recesses of the inner surface. SEM×1500 |
 | Figure 24. The accumulation of strands of fibroin and blood cells in the recesses of the inner surface. SEM×1500 |
 | Figure 25. The accumulation of strands of fibrin and blood cells in the recesses of the inner surface. SEM×1500 |
 | Figure 26. Discocytes and pathological forms of red blood cells, fibrin on the inner surface of a vein. SEM×2000 |
 | Figure 27. Discocytes and pathological forms of red blood cells, fibrin on the inner surface of a vein. SEM×2000 |
 | Figure 28. Discocytes and pathological forms of red blood cells, fibrin on the inner surface of a vein. SEM×3000 |
4. Conclusions
Conducted morphological studies revealed a number of structural changes in the walls of the veins with Varicocele.Firstly, it is the fusion of the walls of the veins of the vaginal (clustered) plexus (pampiniform venous plexus) of the spermatic cord. In this case, a pronounced layer t is absent in the confluence zone. Adventitia.Another feature of the veins is the formation, due to the merger of t. Adventitia, with surrounding connective tissue and adipose tissue of the common venous plexus case.These changes are accompanied by uneven widths of the lumens of the veins in different areas.Another feature of alteration of the walls of the veins with Varicocele is the appearance of bundles of longitudinally located smooth muscle fibers between t. Media and t. Adventitia.Another feature of the veins is the appearance of blood clots in their lumens. Moreover, SEM studies have shown that deposition on the luminal surface of red blood cells and other blood cells with strands of fibrin is the forerunner of blood clots. Moreover, these deposits can be located on the holistic endothelial lining, but are more common in deendothelized areas.Among the identified accumulations of blood cells, erythrocytes dominate, and their pathological forms are echinocytes.Identified features should be considered when conducting surgical treatment of Varicocele.
References
| [1] | Agarwal A., Hamada A., Esteves S. C. Insight into oxidative stress in varicocele-associated male infertility: part 1 //Nature Reviews Urology. – 2012. – Т. 9. – №. 12. – С. 678. |
| [2] | Bittles M. A., Hoffer E. K. Gonadal vein embolization: treatment of varicocele and pelvic congestion syndrome //Seminars in interventional radiology. – © by Thieme Medical Publishers, 2008. – Т. 25. – №. 03. – С. 261-270. |
| [3] | Cortés-Gutiérrez E. I. et al. DNA damage in spermatozoa from infertile men with varicocele evaluated by sperm chromatin dispersion and DBD-FISH //Archives of gynecology and obstetrics. – 2016. – Т. 293. – №. 1. – С. 189-196. |
| [4] | Cho C. L., Esteves S. C., Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation //Asian Journal of Andrology. – 2016. – Т. 18. – №. 2. – С. 186. |
| [5] | Gonda Jr R. L. et al. Diagnosis of subclinical varicocele in infertility //American journal of roentgenology. – 1987. – Т. 148. – №. 1. – С. 71-75. |
| [6] | Liguori G. et al. Color Doppler ultrasound investigation of varicocele //World journal of urology. – 2004. – Т. 22. – №. 5. – С. 378-381. |
| [7] | Marmar J. L., Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data //The Journal of urology. – 1994. – Т. 152. – №. 4. – С. 1127-1132. |
| [8] | Muhitdinovich S. A. et al. Morphologic evaluation of the dilated spermatic veins in children with varicocele // Meditsinskiy vestnik Severnogo Kavkaza. – 2018. – Т. 13. – №. 3. |
| [9] | Muxitdinovich S. A. et al. Scanning electronic microscopy of spermatic veins at varicocele // Dostizheniya nauki i obrazovaniya. – 2017. – №. 9 (22). |
| [10] | Fariello R. M. et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele //Human Reproduction. – 2012. – Т. 27. – №. 11. – С. 3140-3149. |
| [11] | Orhan I. et al. Comparison of two different microsurgical methods in the treatment of varicocele //Archives of andrology. – 2005. – Т. 51. – №. 3. – С. 213-220. |
| [12] | Pastuszak A. W., Wang R. Varicocele and testicular function //Asian journal of andrology. – 2015. – Т. 17. – №. 4. – С. 659. |
| [13] | Piomboni P. et al. Sperm quality improvement after natural anti‐oxidant treatment of asthenoteratospermic men with leukocytospermia //Asian journal of andrology. – 2008. – Т. 10. – №. 2. – С. 201-206. |
| [14] | Redmon J. B., Carey P., Pryor J. L. Varicocele–-the most common cause of male factor infertility? //Human reproduction update. – 2002. – Т. 8. – №. 1. – С. 53-58. |
| [15] | Kısa Ü. et al. Testicular tissue nitric oxide and thiobarbituric acid reactive substance levels: evaluation with respect to the pathogenesis of varicocele //Urological research. – 2004. – Т. 32. – №. 3. – С. 196-199. |
| [16] | Smith R. et al. Increased sperm DNA damage in patients with varicocele: relationship with seminal oxidative stress //Human Reproduction. – 2005. – Т. 21. – №. 4. – С. 986-993. |
| [17] | Sulaymonovich D. S. Ways to Eliminate Postoperative Complications after Ventral Hernia Repair in Patients with Morbid Obesity //American Journal of Medicine and Medical Sciences. – 2017. – Т. 7. – №. 3. – С. 147-150. |
| [18] | Tan S. M. et al. Laparoscopic varicocelectomy: technique and results //British journal of urology. – 1995. – Т. 75. – №. 4. – С. 523-528. |
| [19] | Zini A. et al. Beneficial effect of microsurgical varicocelectomy on human sperm DNA integrity //Human Reproduction. – 2005. – Т. 20. – №. 4. – С. 1018-1021. |
| [20] | Zohdy W., Ghazi S., Arafa M. Impact of varicocelectomy on gonadal and erectile functions in men with hypogonadism and infertility //The journal of sexual medicine. – 2011. – Т. 8. – №. 3. – С. 885-893. |






























 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML